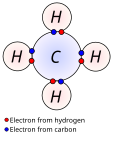
The 18-electron rule is a chemical rule of thumb used primarily for predicting and rationalizing formulas for stable transition metal complexes, especially...
17 KB (1,923 words) - 10:21, 9 April 2025
octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in...
23 KB (2,876 words) - 21:11, 7 May 2025
Tolman's rule states that, in certain chemical reactions, the steps involve exclusively intermediates of 18- and 16 electron configuration. The rule is an...
2 KB (157 words) - 18:16, 6 February 2025
and oxygen, 18-electron rule in inorganic chemistry and organometallic chemistry of transition metals, Hückel's rule for the π-electrons of aromatic compounds...
14 KB (1,629 words) - 15:35, 19 January 2025
This tendency is called the 18-electron rule, because each bonded atom has 18 valence electrons including shared electrons. The heavy group 2 elements...
24 KB (2,333 words) - 15:26, 27 November 2024
shell diagrams. Periodic table (electron configurations) Electron counting 18-electron rule Core charge Re: Why do electron shells have set limits ? madsci...
28 KB (2,765 words) - 11:55, 25 April 2025
are 18 one-sided pentominoes. In the classification of finite simple groups, there are 18 infinite families of groups. The 18-electron rule is a rule of...
6 KB (662 words) - 03:57, 10 May 2025
Aufbau principle (redirect from Principles in distribution of electrons)
'building-up principle'), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available...
28 KB (3,097 words) - 02:28, 13 April 2025
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure)...
60 KB (6,206 words) - 10:35, 24 May 2025
rule, the 18-electron rule, and Hückel's 4n + 2 pi-electron rule are proven to be useful in predicting the molecular stability. Wade's rules were formulated...
14 KB (1,581 words) - 12:51, 25 September 2023
creating a very stable complex, which satisfies the 18-electron rule. The cis-labilization of 18 e− complexes suggests that dissociation of ligand X in...
9 KB (1,053 words) - 14:45, 17 November 2024
Most species with the formula Mx(CO)y follow the 18-electron rule, whereas V(CO)6 has 17 valence electrons. According to the original synthesis by Calderazzo...
5 KB (431 words) - 12:44, 21 July 2024
bonding in transition metal carbonyl complexes which abide by the 18-electron rule, and others arguing the molecule more accurately contains ionic bonds...
22 KB (2,573 words) - 23:22, 22 May 2025
proposed the 16 and 18 electron rule, extending Irving Langmuir's 18-Electron rule to include the many examples of stable 16 electron square planar d8 complexes...
8 KB (898 words) - 17:05, 16 June 2024
available. The formulae of many metal carbonyls can be inferred from the 18-electron rule. Group 2 elements calcium, strontium, and barium can all form octacarbonyl...
71 KB (7,973 words) - 23:53, 15 May 2025
orbital of the ligand. Bridging carbonyl Dewar–Chatt–Duncanson model 18-electron rule Ligand field theory Pi-donor ligands Miessler, Gary L.; Tarr, Donald...
11 KB (1,249 words) - 20:05, 23 May 2025
are the five d, one s and three p orbitals with the corresponding 18-electron rule, spxdy hybridisation is used to model the shape of these molecules...
33 KB (3,204 words) - 20:53, 19 May 2025
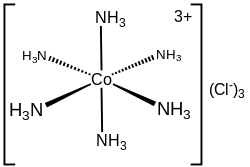
with a low-spin 3d6 octahedral Co(III) center. The cation obeys the 18-electron rule and is considered to be a classic example of an exchange inert metal...
8 KB (665 words) - 21:00, 6 August 2024
ions. The s, p, and d orbitals of the metal can accommodate 18 electrons (see 18-Electron rule). The maximum coordination number for a certain metal is thus...
57 KB (5,547 words) - 11:29, 21 May 2025
which determines the oxidation states. The formula conforms to the 18-electron rule and the complex adopts octahedral geometry with six carbonyl ligands...
18 KB (1,557 words) - 21:05, 22 May 2025
be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18 electrons, whereas the symmetry...
66 KB (8,057 words) - 19:52, 8 May 2025
share their electrons. Apparent violations of the 18-electron rule sometimes are explicable in compounds with unusual hapticities: The 18-VE complex...
13 KB (1,321 words) - 20:45, 16 January 2024
reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy. Many...
41 KB (3,992 words) - 11:54, 22 May 2025
generally be ruled out if the original metal complex fulfilled the 18-electron rule because the two metal–oxygen bonds would exceed the 18 electron rule. The...
9 KB (1,023 words) - 12:12, 9 October 2024
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor when pushed or pulled by...
52 KB (7,204 words) - 22:26, 22 May 2025
enforced by the 18-electron rule, since CpFe(CO)2(η1-C3H5) is already an 18-electron complex, while an η3-allyl ligand would result in an electron count of 20...
13 KB (1,500 words) - 21:37, 23 May 2025
(oxidation state zero) and [Fe(CO) 4]2− (oxidation state −2) in which the 18-electron rule is obeyed. These complexes are also covalent. Ionic compounds are mostly...
40 KB (4,500 words) - 18:28, 24 May 2025
in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities...
31 KB (3,163 words) - 08:57, 10 May 2025
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such...
20 KB (2,190 words) - 16:15, 15 October 2024
complex to comply with the 18-electron rule and maximise stability. The equivalent iron(II) complex undergoes a reversible one-electron reduction (at −0.48 V...
41 KB (4,013 words) - 02:09, 12 May 2025