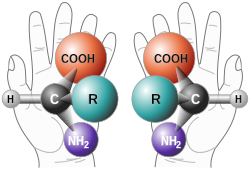
reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis. Asymmetric induction was introduced by Hermann Emil Fischer...
29 KB (3,695 words) - 13:28, 30 January 2025
Enantioselective synthesis (redirect from Asymmetric catalyst)
through interactions at the transition state. This biasing is known as asymmetric induction and can involve chiral features in the substrate, reagent, catalyst...
41 KB (4,751 words) - 14:29, 25 May 2025
effect, change in electron density Asymmetric induction, preferring one stereoisomer over another Grammar induction Inductive bias Inductive probability...
2 KB (199 words) - 11:02, 1 April 2025
Ene reaction (section Internal asymmetric induction)
carbonyl groups. The catalysts were found to afford high levels of asymmetric induction in several processes, including the ene reaction of ethyl glyoxylate...
25 KB (3,164 words) - 18:29, 25 May 2025
C2-Symmetric ligands (redirect from Asymmetric ligand)
used in Sharpless asymmetric dihydroxylation While the presence of any symmetry element within a ligand intended for asymmetric induction might appear counterintuitive...
11 KB (1,175 words) - 10:25, 15 March 2025
Sharpless asymmetric dihydroxylation (also called the Sharpless bishydroxylation) is the chemical reaction of an alkene with osmium tetroxide in the presence...
20 KB (2,189 words) - 15:19, 2 December 2024
Tsuji–Trost reaction (redirect from Trost asymmetric allylic alkylation)
Asymmetric induction in allylic alkylations." J. Am. Chem. Soc. 1973, 95, 8200–8201. doi:10.1021/ja00805a056. Trost, B. M.; Strege, P. E. "Asymmetric...
24 KB (2,775 words) - 15:06, 26 September 2024
Chiral auxiliary (redirect from Myers' asymmetric alkylation)
"Preparation of an Optically Active Prostaglandin Intermediate via Asymmetric Induction". J. Am. Chem. Soc. 97 (23): 6908–6909. Bibcode:1975JAChS..97.6908C...
34 KB (3,726 words) - 10:21, 15 March 2025
Asymmetric hydrogenation is a chemical reaction that adds two atoms of hydrogen to a target (substrate) molecule with three-dimensional spatial selectivity...
65 KB (6,424 words) - 14:52, 26 May 2025
predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center...
15 KB (1,587 words) - 23:07, 24 December 2024
structure. He also did work in stereochemistry and Cram's rule of asymmetric induction is named after him. In 1973, Cram collaborated on research with Irish...
16 KB (1,323 words) - 13:04, 11 September 2024
chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound...
24 KB (2,639 words) - 16:47, 23 May 2025
Phosphinooxazolines (section Asymmetric Hydrogenation)
Bunt, Richard C. (2012). "Investigation of the Electronic Origin of Asymmetric Induction in Palladium-Catalyzed Allylic Substitutions with Phosphinooxazoline...
10 KB (1,075 words) - 23:43, 29 June 2024
transfer of an H2 molecule from the reductant to the substrate. Asymmetric induction in transition metal catalyzed reactions is achieved through the use...
12 KB (1,406 words) - 20:37, 22 July 2024
employing chiral starting materials have been used to effect either asymmetric induction or simple diastereoselection (1) After carbanion formation, the [2...
13 KB (1,449 words) - 05:36, 17 December 2024
2010. Other chiral additives can be used as the initial source of asymmetric induction, with the major product of that first reaction being rapidly amplified...
6 KB (640 words) - 10:11, 15 March 2025
1,3-Dipolar cycloaddition (section Stereoselectivity and asymmetric induction of the 1,3-dipolar cycloaddition reaction mediated by metal catalysis of diazocarbonyl compounds)
in particular Rh2[(S)-DOSP]4 and Rh2[(S)-BPTV]4 can induce modest asymmetric induction and was used to synthesize the antifungal agent pseudolaric acid...
65 KB (6,396 words) - 07:16, 24 May 2025
= OR*). (1) Asymmetric versions of the above reaction have taken advantage of a number of strategies for achieving asymmetric induction. The highest...
14 KB (1,634 words) - 16:11, 7 March 2021
enantioselective way. Ruppert's reagent has been used for this purpose in an asymmetric induction approach to functionalise chiral amino acid derivates, saccharides...
43 KB (4,098 words) - 19:12, 25 April 2025
chiral chemical synthesis, and specifically of the phenomenon of asymmetric induction during nucleophilic attack at hindered carbonyl centers (see the...
15 KB (1,606 words) - 10:46, 29 April 2025
potential application is its use as a temporary substituent promoting asymmetric induction for example in this diastereoselective one-pot reaction involving...
9 KB (1,016 words) - 16:11, 30 May 2025
et al. (1981). "Asymmetric total synthesis of erythromcin. 1. Synthesis of an erythronolide A secoacid derivative via asymmetric induction". J. Am. Chem...
17 KB (1,728 words) - 19:25, 25 May 2025
(see below) In 2004 Node obtained (−)-galanthamine via a remote asymmetric induction method with starting chiral compound D-phenylalanine. Brown prepared...
15 KB (1,662 words) - 02:08, 18 September 2024
"Studies in Stereochemistry. X. The Rule of "Steric Control of Asymmetric Induction" in the Syntheses of Acyclic Systems". Journal of the American Chemical...
3 KB (339 words) - 14:03, 16 April 2025
(3 January 2014). "Foldamer-Mediated Remote Stereocontrol: >1,60 Asymmetric Induction" (PDF). Angewandte Chemie International Edition. 53 (1): 151–155...
6 KB (401 words) - 15:58, 22 May 2025
the imine, which generally results in a trans product. Reviews on asymmetric induction of the Staudinger synthesis, including the use of organic and organometallic...
10 KB (1,112 words) - 04:32, 19 August 2024
discrimination between these possible enantiomers that requires asymmetric induction. Torquoselectivity is also used to describe selective electrocyclic...
4 KB (382 words) - 10:33, 15 March 2025
Chiral Lewis acid (section Asymmetric synthesis)
one enantiomer or diastereomer over the other is formally known as asymmetric induction. In this kind of Lewis acid, the electron-accepting atom is typically...
11 KB (1,261 words) - 19:02, 22 May 2025
rearrangement Coupling reaction Crabbé reaction Craig method Cram's rule of asymmetric induction Creighton process Criegee reaction Criegee rearrangement Cross metathesis...
37 KB (3,428 words) - 16:27, 11 November 2024
Etter, Jeffrey B. (1987). "Lanthanides in organic synthesis. 8. 1.3-Asymmetric induction in intramolecular Reformatskii-type reactions promoted by samarium...
13 KB (1,275 words) - 23:31, 13 March 2025