In quantum mechanics, the azimuthal quantum number ℓ is a quantum number for an atomic orbital that determines its orbital angular momentum and describes...
19 KB (2,143 words) - 08:39, 24 May 2025
atom, four quantum numbers are needed. The traditional set of quantum numbers includes the principal, azimuthal, magnetic, and spin quantum numbers. To...
30 KB (3,266 words) - 03:49, 7 June 2025
used to describe the quantum state of an electron in an atom are the principal quantum number n, the azimuthal (orbital) quantum number ℓ {\displaystyle \ell...
10 KB (1,196 words) - 19:04, 21 November 2024
{\displaystyle n=n_{r}+\ell +1} where ℓ is the azimuthal quantum number and nr is equal to the number of nodes in the radial wavefunction. The definite...
8 KB (1,135 words) - 13:04, 26 February 2025
(astronomy) Angular displacement Angzarr (⍼) Azimuthal quantum number Azimuthal equidistant projection Azimuth recording Bearing (navigation) Clock position Course...
14 KB (1,768 words) - 08:17, 23 June 2025
from the spectroscopic notation for the value of an electron's azimuthal quantum number: sharp (0), principal (1), diffuse (2), and fundamental (3). Succeeding...
19 KB (2,487 words) - 03:05, 25 July 2025
\ell +s} where ℓ is the azimuthal quantum number (parameterizing the orbital angular momentum) and s is the spin quantum number (parameterizing the spin)...
3 KB (351 words) - 19:01, 23 April 2024
four of their quantum numbers, which are: n, the principal quantum number; ℓ, the azimuthal quantum number; mℓ, the magnetic quantum number; and ms, the...
26 KB (3,470 words) - 01:14, 27 July 2025
Slater's rules (category Quantum chemistry)
in order of increasing principal quantum number n, and for equal n in order of increasing azimuthal quantum number l, except that s- and p- orbitals...
8 KB (1,255 words) - 12:33, 19 July 2022
1 , 2 , 3 … {\displaystyle n=1,2,3\ldots } The next quantum number, the azimuthal quantum number, denoted l, describes the shape of the orbital. The shape...
77 KB (9,422 words) - 13:00, 17 July 2025
Aufbau principle (category Foundational quantum physics)
the principal quantum number. The maximum number of electrons in a subshell is equal to 2(2l + 1), where the azimuthal quantum number l is equal to 0...
28 KB (3,099 words) - 19:02, 17 June 2025
usage. The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information...
46 KB (8,376 words) - 04:09, 2 August 2025
Bargmann's limit (category Quantum mechanics)
the number N ℓ {\displaystyle N_{\ell }} of bound states with azimuthal quantum number ℓ {\displaystyle \ell } in a system with central potential V {\displaystyle...
5 KB (1,046 words) - 14:13, 3 July 2022
identified by letters. These letters were later associated with the azimuthal quantum number, ℓ. The letters, "s", "p", "d", and "f", for the first four values...
6 KB (638 words) - 01:53, 27 December 2024
usage. The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information...
52 KB (8,705 words) - 16:03, 28 July 2025
Periodic table (section Atomic number)
by the quantum numbers. Four numbers describe an orbital in an atom completely: the principal quantum number n, the azimuthal quantum number ℓ (the orbital...
251 KB (27,139 words) - 04:45, 30 July 2025
Arnold Sommerfeld (category German quantum physicists)
He introduced the second quantum number, azimuthal quantum number, and the third quantum number, magnetic quantum number. He also introduced the fine-structure...
56 KB (6,094 words) - 17:35, 6 July 2025
dipole can assume a number of discrete energy eigenstates, depending on the value of its angular momentum (azimuthal) quantum number. The oscillating field...
20 KB (3,284 words) - 01:23, 17 December 2024
Computational chemists see the split as a change of the second (azimuthal) quantum number ℓ from 1 to 1⁄2 and 3⁄2 for the more stabilized and less stabilized...
74 KB (12,027 words) - 23:50, 18 June 2025
The old quantum theory is a collection of results from the years 1900–1925, which predate modern quantum mechanics. The theory was never complete or self-consistent...
33 KB (4,834 words) - 07:37, 20 July 2025
Energy level (redirect from Quantum energy level)
{eff}}}^{2}}{n^{2}}}} In such cases, the orbital types (determined by the azimuthal quantum number ℓ) as well as their levels within the molecule affect Zeff and...
23 KB (2,871 words) - 17:36, 27 July 2025
fusion). The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information...
55 KB (9,455 words) - 20:18, 21 July 2025
level. The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information...
70 KB (11,063 words) - 03:00, 31 July 2025
usage. The quantum number corresponds to the letter in the electron orbital name: 0 to s, 1 to p, 2 to d, etc. See azimuthal quantum number for more information...
49 KB (8,547 words) - 12:21, 15 May 2025
Electron shell (category Quantum mechanics)
second column is the azimuthal quantum number (ℓ) of the subshell. The precise definition involves quantum mechanics, but it is a number that characterizes...
28 KB (2,765 words) - 11:55, 25 April 2025
Wave function (redirect from Quantum wave function)
the principal quantum number, ℓ = 0, 1, ..., n − 1 the azimuthal quantum number, m = −ℓ, −ℓ + 1, ..., ℓ − 1, ℓ the magnetic quantum number. Hydrogen-like...
99 KB (13,584 words) - 18:24, 21 June 2025
describes the average distance of an electron from the nucleus. The azimuthal quantum number l {\displaystyle l} describes the relative shape of the region...
15 KB (2,641 words) - 09:24, 14 February 2025
Atomic orbital (category Quantum chemistry)
density vanishes. The number of nodal surfaces is controlled by the quantum numbers n and ℓ. An orbital with azimuthal quantum number ℓ has ℓ radial nodal...
84 KB (10,940 words) - 15:49, 28 July 2025
Angular momentum operator (redirect from Angular momentum (quantum mechanics))
values are characterized by the azimuthal quantum number (l) and the magnetic quantum number (m). In this case the quantum state of the system is a simultaneous...
43 KB (6,728 words) - 16:08, 29 July 2025
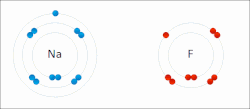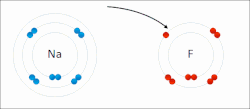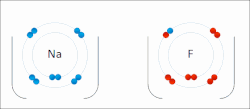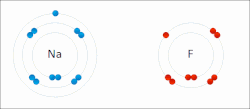
configuration Aufbau principle Quantum numbers Azimuthal quantum number Principal quantum number Magnetic quantum number Spin quantum number "Ionic bond". IUPAC...
18 KB (2,340 words) - 19:04, 15 June 2025