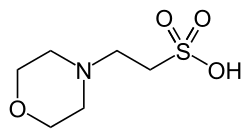
A buffer solution is a solution where the pH does not change significantly on dilution or if an acid or base is added at constant temperature. Its pH...
22 KB (2,336 words) - 05:58, 25 May 2025
Phosphate-buffered saline (PBS) is a buffer solution (pH ~ 7.4) commonly used in biological research. It is a water-based salt solution containing disodium...
9 KB (839 words) - 15:03, 11 May 2025
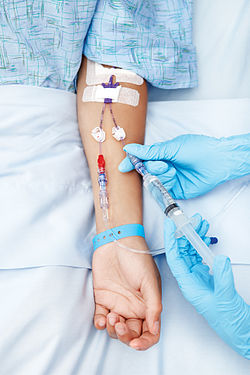
Intravenous therapy (redirect from Intravenous buffer solution)
base solution to which medications are added also has some buffering effect. Another solution administered intravenously as a buffering solution is sodium...
60 KB (7,182 words) - 17:00, 18 June 2025
A lysis buffer is a buffer solution used for the purpose of breaking open cells for use in molecular biology experiments that analyze the labile macromolecules...
15 KB (1,825 words) - 02:30, 26 May 2025
Producer–consumer problem (redirect from Bounded-buffer problem)
known as the bounded-buffer problem) is a family of problems described by Edsger W. Dijkstra since 1965. Dijkstra found the solution for the producer-consumer...
17 KB (2,183 words) - 06:50, 21 June 2025
TE buffer is a commonly used buffer solution in molecular biology, especially in procedures involving DNA, cDNA or RNA. "TE" is derived from its components:...
4 KB (552 words) - 15:35, 7 July 2023
Tris/Borate/EDTA, is a buffer solution containing a mixture of Tris base, boric acid and EDTA. In molecular biology, TBE and TAE buffers are often used in...
3 KB (357 words) - 20:28, 18 June 2025
PH (redirect from Neutral solution)
standard solution, and the reading on a pH meter is adjusted to be equal to the standard buffer's value. The reading from a second standard buffer solution is...
53 KB (6,540 words) - 15:50, 17 June 2025
TAE buffer is a buffer solution containing a mixture of Tris base, acetic acid and EDTA. In molecular biology, it is used in agarose electrophoresis typically...
4 KB (587 words) - 05:03, 24 June 2025
McIlvaine buffer is a buffer solution composed of citric acid and disodium hydrogen phosphate, also known as citrate-phosphate buffer. It was introduced...
3 KB (199 words) - 00:27, 16 March 2025
value of 6.15 at 20 °C. The pH (and pKa at ionic strength I≠0) of the buffer solution changes with concentration and temperature, and this effect may be...
6 KB (475 words) - 01:46, 24 February 2024
Henderson–Hasselbalch equation (redirect from Buffer equation)
Henderson-Hasselbalch equation is often used for estimating the pH of buffer solutions by approximating the actual concentration ratio as the ratio of the...
18 KB (2,395 words) - 00:21, 24 June 2025
to make buffer solutions. It has a pKa value of 7.550 (I=0, 25°C). It is one of the Good's buffers and can be used to make buffer solutions in the pH...
2 KB (90 words) - 16:04, 6 June 2025
biochemistry and molecular biology, saline-sodium citrate (SSC) buffer is used as a hybridization buffer, to control stringency for washing steps in protocols for...
992 bytes (101 words) - 22:18, 16 January 2022
vial and capillary are filled with an electrolyte such as an aqueous buffer solution. To introduce the sample, the capillary inlet is placed into a vial...
36 KB (4,675 words) - 06:08, 26 May 2025
commonly used to make buffer solutions. It can bind divalent cations, including Co(II) and Ni(II). TAPS is effective to make buffer solutions in the pH range...
3 KB (174 words) - 01:42, 11 February 2024
Sodium acetate (section Buffer solution)
eat at low concentration. A solution of sodium acetate (a basic salt of acetic acid) and acetic acid can act as a buffer to keep a relatively constant...
17 KB (1,245 words) - 14:37, 11 June 2025
Total ionic strength adjustment buffer (TISAB) is a buffer solution which increases the ionic strength of a solution to a relatively high level. This is...
3 KB (478 words) - 17:46, 28 May 2022
Good's buffers (also Good buffers) are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues...
8 KB (860 words) - 20:23, 3 June 2025
Ringer's solution typically contains sodium chloride, potassium chloride, calcium chloride and sodium bicarbonate, with the last used to buffer the pH....
6 KB (616 words) - 08:40, 9 June 2025
vary, and the resulting solution would still be referred to as "borate buffered saline". Borate concentration (giving buffering capacity) can vary from...
2 KB (278 words) - 15:05, 11 May 2025
Tris (redirect from Tris buffer)
and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary...
12 KB (1,069 words) - 00:07, 24 June 2025
Robinson (1904–1979). Buffer solution Good's buffers Mongay, Carlos; Cerdà, Víctor (January 1974). "A Britton-Robinson Buffer of Known Ionic Strength"...
2 KB (278 words) - 00:46, 26 May 2025
Acid–base homeostasis (redirect from Body buffer)
occurred. But buffers cannot correct abnormal pH levels in a solution, be that solution in a test tube or in the extracellular fluid. Buffers typically consist...
21 KB (2,428 words) - 15:03, 20 November 2024
The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO− 3), and carbon...
14 KB (1,725 words) - 02:43, 6 April 2025
One use of conjugate acids and bases lies in buffering systems, which include a buffer solution. In a buffer, a weak acid and its conjugate base (in the...
13 KB (1,310 words) - 14:02, 17 May 2025
chemistry, a knowledge of pKa values is necessary for the preparation of buffer solutions and is also a prerequisite for a quantitative understanding of the...
103 KB (11,534 words) - 07:28, 2 June 2025
SB buffer is a buffer solution used in agarose and polyacrylamide gel electrophoresis for the separation of nucleic acids such as DNA and RNA. "SB" is...
2 KB (308 words) - 22:18, 16 January 2022
regulation of hydrogen ion concentration by a pH buffer A metal-ion buffer solution contains the free (hydrated) metal ion along with a complex compound...
1 KB (177 words) - 23:22, 12 October 2023
CHES (N-cyclohexyl-2-aminoethanesulfonic acid) is a buffering agent. CHES buffers have a useful range of pH 8.6–10. It typically appears as a white crystalline...
2 KB (117 words) - 18:20, 7 June 2025