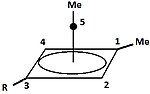
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium CH+ 3, methanium CH+ 5 and vinyl C 2H+ 3...
42 KB (5,038 words) - 07:48, 9 March 2024
Reaction intermediate (section Carbocations)
isolated. Cations, often carbocations, serve as intermediates in various types of reactions to synthesize new compounds. Carbocations are formed in two major...
14 KB (1,830 words) - 09:50, 26 May 2024
accurately described using steady-state kinetics. The reaction involves a carbocation intermediate and is commonly seen in reactions of secondary or tertiary...
15 KB (1,791 words) - 04:33, 29 May 2024
in an addition reaction to form an alcohol which involve formation of carbocations. The hydroxyl group (OH) bonds to the carbon that has the greater number...
9 KB (1,129 words) - 04:58, 22 February 2024
Tertiary carbon (section Carbocation Stability)
density with the central carbocation to stabilize it. Additionally, the surrounding sp3 hybridized carbons can stabilize the carbocation through hyperconjugation...
7 KB (630 words) - 11:25, 11 February 2024
alkylating agents (ones for which carbocation rearrangement is degenerate), or alkylating agents that yield stabilized carbocations (e.g., benzylic or allylic...
20 KB (1,988 words) - 00:39, 15 February 2024
lab at Case Western Reserve University, and has been used to stabilize carbocations and hypercoordinated carbonium ions in liquid media. Magic acid and other...
15 KB (1,711 words) - 07:31, 25 April 2024
A pyramidal carbocation is a type of carbocation with a specific configuration. This ion exists as a third class, besides the classical and non-classical...
27 KB (2,648 words) - 23:20, 18 March 2024
involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic...
12 KB (1,456 words) - 21:30, 14 April 2024
phenyl carbocation > hydride > tertiary carbocation (if formed by migration) > secondary carbocation (if formed by migration) > methyl carbocation. {Why...
8 KB (969 words) - 05:35, 16 January 2024
stabilize the charge on the carbocation through resonance and distribution of charge. In this case, tertiary carbocation will react faster than a secondary...
12 KB (1,436 words) - 11:12, 17 April 2024
Rearrangement reaction (redirect from Carbocation rearrangement)
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural...
5 KB (595 words) - 23:43, 15 February 2024
(proton) attacks the double bond forming a carbocation, which then reacts with the nucleophile (bromine). The carbocation can be formed on either side of the...
66 KB (8,031 words) - 11:55, 4 June 2024
of the corresponding carbocations. Tertiary carbocations are far more stable than secondary carbocations, and primary carbocations are the least stable(due...
4 KB (464 words) - 12:16, 21 March 2024
tert-butanol is a tertiary alcohol, the relative stability of the tert-butyl carbocation in the step 2 allows the SN1 mechanism to be followed, whereas a primary...
5 KB (229 words) - 19:33, 27 October 2022
Polyvinyl chloride (PVC), a particular vinyl polymer Vinyl cation, a type of carbocation Vinyl group, a broad class of organic molecules in chemistry Vinyl polymer...
1 KB (189 words) - 02:18, 22 July 2023
deprotonation of the α-carbon. This results in the formation of a carbocation intermediate. The carbocation is then deprotonated resulting in the formation of a new...
17 KB (2,146 words) - 09:44, 22 May 2024
stabilizing carbocation formation, the fragmentation becomes a viable reaction pathway. The reaction generates a nitrile and a carbocation, which is quickly...
14 KB (1,419 words) - 04:50, 28 December 2023
Triphenylcarbenium (redirect from Trityl carbocation)
2019-07-25. Naidu, Veluru Ramesh; Ni, Shengjun; Franzén, Johan (2015). "The Carbocation: A Forgotten Lewis Acid Catalyst". ChemCatChem. 7 (13): 1896–1905. doi:10...
4 KB (426 words) - 05:25, 30 April 2024
Nonclassical ion (redirect from Non-classical carbocation)
Nonclassical carbocations are stabilized by charge delocalization from contributions of neighbouring C−C or C−H bonds, which can form bridged intermediates...
4 KB (418 words) - 10:12, 12 March 2023
limonene is formed from geranyl pyrophosphate, via cyclization of a neryl carbocation or its equivalent as shown. The final step involves loss of a proton...
19 KB (1,519 words) - 20:48, 19 May 2024
leaving group, this generates the carbocation. In the second step, the chloride nucleophile attacks the carbocation to form the product. 2-Chlorobutane...
7 KB (755 words) - 06:57, 17 February 2024
stability of the carbocation, and steric effects. As brief examples, the formation of a sterically unencumbered, stabilized carbocation favors the AdE2...
19 KB (2,321 words) - 12:01, 2 March 2024
trans-fused carbon ring systems formed by a carbocation mediated cyclization. The remaining tertiary carbocation is quenched by a molecule of water. After...
10 KB (907 words) - 03:42, 3 May 2024
from Higashimura, Sawamoto and Kennedy. Typically, generating a stable carbocation for a prolonged period of time is difficult, due to the possibility for...
37 KB (4,445 words) - 05:32, 3 June 2024
Ionization: the carbon-halogen bond breaks to give a carbocation intermediate. deprotonation of the carbocation. E1 typically takes place with tertiary alkyl...
14 KB (1,844 words) - 22:24, 27 May 2024
charge in an organic ion is formally centred on a carbon, it is termed a carbocation (if positively charged) or carbanion (if negatively charged). Monatomic...
30 KB (3,020 words) - 20:58, 7 May 2024
A Wagner–Meerwein rearrangement is a class of carbocation 1,2-rearrangement reactions in which a hydrogen, alkyl or aryl group migrates from one carbon...
6 KB (633 words) - 02:17, 2 December 2023
reactivity of carbocations via superacids. For this research, Olah was awarded a Nobel Prize in Chemistry in 1994 "for his contribution to carbocation chemistry...
17 KB (1,448 words) - 12:12, 11 May 2024
Hydrocarbons may form charged structures: positively charged carbocations or negative carbanions. Carbocations are often named -um. Examples are tropylium and triphenylmethyl...
31 KB (1,212 words) - 22:07, 3 June 2024