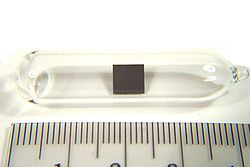
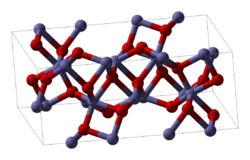
Cerium(III) oxalate (cerous oxalate) is the inorganic cerium salt of oxalic acid. It is a white crystalline solid with the chemical formula of Ce2(C2O4)3...
4 KB (189 words) - 16:00, 24 December 2023
elements resist oxidation. Cerium(IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide. Cerium also forms cerium(III) oxide, Ce 2O 3...
24 KB (2,374 words) - 11:12, 10 April 2025
Rare-earth element (redirect from Cerium group)
these elements. They are relatively plentiful in the entire Earth's crust (cerium being the 25th-most-abundant element at 68 parts per million, more abundant...
156 KB (16,714 words) - 14:48, 1 May 2025
Gadolinium-doped ceria (category Cerium(IV) compounds)
and nanocasting using cerium sources such as cerium nitrate, ceric ammonium nitrate, cerium oxalate, cerium carbonate and cerium hydroxide. GDC has been...
5 KB (613 words) - 14:02, 10 April 2025
Cerium is a chemical element; it has symbol Ce and atomic number 58. It is a soft, ductile, and silvery-white metal that tarnishes when exposed to air...
51 KB (6,075 words) - 15:55, 11 April 2025
Oxalate sulfates are mixed anion compounds containing oxalate and sulfate. They are mostly transparent, and any colour comes from the cations. Related...
40 KB (1,830 words) - 15:13, 11 December 2024
with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate...
48 KB (6,053 words) - 00:23, 6 May 2025
Palonosetron A04AA55 Palonosetron, combinations A04AD01 Scopolamine A04AD02 Cerium oxalate A04AD04 Chlorobutanol A04AD05 Metopimazine A04AD10 Dronabinol A04AD11...
860 bytes (221 words) - 06:20, 31 December 2022
Ceric ammonium nitrate (redirect from Ammonium cerium(IV) nitrate)
compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised oxidizing agent in organic synthesis and a standard...
11 KB (894 words) - 15:29, 13 November 2024
Cerium(III) iodide – CeI3 Cerium(III) nitrate – Ce(NO3)3 Cerium(III) oxide – Ce2O3 Cerium(III) sulfate – Ce2(SO4)3 Cerium(III) sulfide – Ce2S3 Cerium(IV)...
121 KB (8,924 words) - 00:00, 18 May 2025
Sodium chloride (common table salt), developed by silver nitrate. Cerium oxalate developed by manganese sulfate and hydrogen peroxide Some inks glow...
27 KB (3,291 words) - 16:46, 19 May 2025
Atropine Bismuth subcarbonate Bismuth subnitrate Caffeine Charcoal Cerium oxalate Chalk Emetine Lactic acid fermentation Ferrous carbonate Arsenic Iron...
23 KB (2,907 words) - 21:33, 26 August 2024
6/mcm), cell parameters a = 0.7078 nm, c = 0.7239 nm, Z = 6, structure like cerium(III) fluoride (CeF3). Praseodymium(III) fluoride is a green, odourless,...
6 KB (514 words) - 15:03, 8 February 2025
lanthanum and cerium in oxide and carbonate form, and that of neodymium-praseodymium, samarium, gadolinium and yttrium in oxide and oxalate form (with more...
20 KB (1,924 words) - 13:13, 25 April 2025
called didymium from a residue he called "lanthana", in turn separated from cerium salts. In 1885, the Austrian chemist Carl Auer von Welsbach separated didymium...
38 KB (5,038 words) - 17:38, 4 May 2025
the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing....
33 KB (4,000 words) - 08:45, 8 May 2025
and G H I L M N and O P R S T U, V, and X Y Z References External links "Cerium (III) Acetate" (PDF). Retrieved 2019-09-28. "Magnesium Sulfite" (PDF). srdata...
83 KB (225 words) - 19:01, 4 May 2025
Ammonium cerium(IV) sulfate is an inorganic compound with the formula (NH4)4Ce(SO4)4·2H2O. It is an orange-colored solid. It is a strong oxidant, the potential...
3 KB (140 words) - 13:20, 20 January 2024
substance in the laboratory, by controlled heating of tin(II) oxalate (stannous oxalate) in the absence of air or under a CO2 atmosphere. This method...
10 KB (912 words) - 13:29, 13 April 2025
mainly produced by calcination of plutonium(IV) oxalate, Pu(C2O4)2·6H2O, at 300 °C. Plutonium oxalate is obtained during the reprocessing of nuclear fuel...
13 KB (1,257 words) - 21:04, 26 February 2025
the solution is treated with ammonium oxalate to convert rare earths in to their insoluble oxalates. The oxalates are converted to oxides by annealing...
14 KB (1,188 words) - 09:13, 3 May 2025
praseodymium(III) oxalate, are used to colour some glasses and enamels. If mixed with certain other materials, praseodymium(III) oxalate paints glass intense...
17 KB (1,698 words) - 04:54, 4 April 2025
it lies to the right of actinium, to the left of protactinium, and below cerium. Pure thorium is very ductile and, as normal for metals, can be cold-rolled...
146 KB (16,633 words) - 08:29, 14 May 2025
ionization chambers. After a test, dispersed La-140 rapidly decays into stable cerium-140, reducing the radiation hazard for the operators after several half-lives...
29 KB (3,840 words) - 11:55, 9 December 2024
the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing....
34 KB (4,239 words) - 03:04, 24 March 2025
white solid. Yttrium forms a water-insoluble fluoride, hydroxide, and oxalate, but its bromide, chloride, iodide, nitrate and sulfate are all soluble...
58 KB (6,693 words) - 23:04, 17 May 2025
ultrafine particles can be prepared by thermal decomposition of iron(III) oxalate. Several other phases have been identified or claimed. The beta phase (β-phase)...
23 KB (2,154 words) - 22:43, 12 February 2025
Pm2(SO4)3·8H2O is 2.86 g/cm3. The oxalate, Pm2(C2O4)3·10H2O, has the lowest solubility of all lanthanide oxalates. Unlike the nitrate, the oxide is similar...
42 KB (4,976 words) - 01:40, 11 May 2025
Also compounds containing C=O bonds have higher levels. These include oxalates, squarates and cyanurates. One trade-off is with band gap. If the band...
28 KB (2,299 words) - 11:57, 10 March 2025
the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing....
24 KB (2,487 words) - 20:20, 3 January 2024