Chromate salts contain the chromate anion, CrO2−4. Dichromate salts contain the dichromate anion, Cr2O2−7. They are oxyanions of chromium in the +6 oxidation...
13 KB (1,261 words) - 18:39, 24 May 2025
Subsequent to its formation, the chromate salt is converted to sodium dichromate, the precursor to most chromium compounds and materials. The industrial route...
7 KB (583 words) - 15:47, 11 March 2025
containing dichromate ions, a yellow solution is obtained due to the formation of chromate ions (CrO2−4). For example, potassium chromate is produced...
18 KB (1,786 words) - 15:49, 4 June 2025
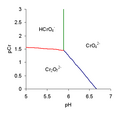
line to be drawn on the predominance diagram. Red line Hydrogen chromate and dichromate have equal concentrations. Setting [HCrO−4] equal to [Cr2O2−7]...
5 KB (607 words) - 13:59, 17 August 2024
potassium and sodium dichromates are very similar. When treated with lead(II) nitrate, it gives an orange-yellow precipitate, lead(II) chromate. Unlike...
6 KB (487 words) - 20:54, 4 June 2025
around 30 to 35 degrees C Dichromate and chromate salts are oxidizing agents. For the tanning of leather, sodium dichromate is first reduced with sulfur...
9 KB (738 words) - 18:06, 4 February 2025
solution consisting of 182 g/L sodium dichromate (Na2Cr2O7 · 2H2O) and 6 mL/L concentrated sulfuric acid. The chromate coating process starts with a redox...
15 KB (1,791 words) - 04:34, 9 March 2025
create zinc chromate for use in industry. This process is done by putting zinc or a zinc plated metal in a solution of sodium dichromate and sulfuric acid...
10 KB (886 words) - 13:17, 17 June 2024
Ammonium dichromate is an inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6...
13 KB (1,072 words) - 08:46, 24 May 2025
Chromate or chromat, and their derived terms, may refer to: Chromate and dichromate, ions Monochromate, an ion Trichromate, an ion Tetrachromate, an ion...
911 bytes (123 words) - 23:29, 3 June 2021
lead chromate are known, orthorhombic and the more stable monoclinic form. Monoclinic lead chromate is used in paints under the name chrome yellow, and many...
11 KB (980 words) - 21:18, 29 May 2025
Chromic acid (category Chromates)
solution formed by the addition of sulfuric acid to aqueous solutions of dichromate. It consists at least in part of chromium trioxide. The term "chromic...
15 KB (1,370 words) - 12:54, 5 May 2025
Chromium (section Chemistry and compounds)
temperatures dissolves the chromates and leaves the insoluble iron oxide. The chromate is converted by sulfuric acid into the dichromate. 4 FeCr2O4 + 8 Na2CO3...
113 KB (12,319 words) - 11:03, 12 June 2025
chloride and sodium chromate, or from strontium carbonate and sodium dichromate. Strontium chromate is approximately 30 times more soluble in water at 100 °C...
4 KB (233 words) - 15:33, 13 March 2025
Hexavalent chromium (section Occurrence and uses)
sodium dichromate. Sodium chromate is converted into other hexavalent chromium compounds such as chromium trioxide and various salts of chromate and dichromate...
66 KB (7,085 words) - 03:45, 20 May 2025
Chromium(VI) compounds are oxidants at low or neutral pH. Chromate anions (CrO2− 4) and dichromate (Cr2O72−) anions are the principal ions at this oxidation...
14 KB (1,400 words) - 06:14, 26 May 2025
Barium chromate, is a yellow sand like powder with the formula BaCrO4. It is a known oxidizing agent and produces a green flame when heated, a result...
9 KB (1,013 words) - 16:23, 19 February 2025
Ammonium chromate is a salt with the formula (NH4)2CrO4. It forms yellow, monoclinic crystals; made from ammonium hydroxide and ammonium dichromate; used...
3 KB (151 words) - 14:43, 24 March 2025
Chromium(III) oxide (redirect from Chromium(III) chromate)
Heating with chlorine and carbon yields chromium(III) chloride and carbon monoxide: Cr 2O 3 + 3 Cl 2 + 3 C → 2 CrCl 3 + 3 CO Chromates salts form by the oxidation...
10 KB (948 words) - 16:05, 25 April 2025
containing the element chlorine. Chromate Chromate salts contain the chromate anion, CrO2− 4. Dichromate salts contain the dichromate anion, Cr 2O2− 7. They are...
279 KB (31,753 words) - 07:09, 28 January 2025
dichromate solution. This is followed by drying and immersion in a 2% aqueous silver nitrate solution. By the same reaction as above, silver chromate...
14 KB (1,241 words) - 02:10, 13 May 2025
dichromate) yields the chromate following alkalinisation with caesium hydroxide: Cs2Cr2O7(aq) + 2 CsOH(aq) → 2 Cs2CrO4(aq) + H2O(ℓ) Caesium chromate was...
5 KB (390 words) - 01:26, 24 May 2025
dichromic acids and chromium trioxide, pyridinium chlorochromate (PCC), and chromate/dichromate compounds such as Sodium dichromate (Na2Cr2O7) Permanganate...
9 KB (889 words) - 10:32, 20 April 2025
Pyridinium chlorochromate (category Chromates)
lupenone: With tertiary alcohols, the chromate ester formed from PCC can isomerize via a [3,3]-sigmatropic reaction and following oxidation yield an enone...
10 KB (780 words) - 19:45, 11 April 2025
Iron(III) chromate is the iron(III) salt of chromic acid with the chemical formula Fe2(CrO4)3. Iron(III) chromate was discovered by Samuel Hibbert-Ware...
2 KB (127 words) - 16:33, 24 December 2024
is a powerful oxidiser, a mutagen, and a carcinogen. Chromium trioxide is generated by treating sodium dichromate with sulfuric acid: H2SO4 + Na2Cr2O7...
11 KB (810 words) - 18:07, 4 February 2025
Calcium chromate is an inorganic compound with the formula CaCrO4, i.e. the chromate salt of calcium. It is a bright yellow solid which is normally found...
6 KB (399 words) - 14:22, 10 January 2024
important industrial uses. This chromate can be manufactured as a powder. Before 1940, the literature about magnesium chromate and its hydrates was sparse, but...
4 KB (366 words) - 09:49, 21 June 2024
of the catalytic mechanism. The active catalyst is often depicted as a chromate ester bound to the silica surface. The mechanism for the polymerization...
3 KB (293 words) - 18:33, 10 December 2024
Cadmium chromate is the inorganic compound with the formula CdCrO4. It is relevant to chromate conversion coating, which is used to passivate common metal...
2 KB (154 words) - 15:07, 24 March 2025