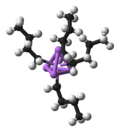
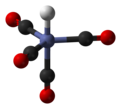
The covalent bond classification (CBC) method, also referred to as LXZ notation, is a way of describing covalent compounds such as organometallic complexes...
6 KB (734 words) - 09:09, 9 January 2024
coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the...
10 KB (1,317 words) - 15:12, 21 May 2025
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared...
29 KB (3,858 words) - 17:32, 1 July 2025
It is classified as an L ligand in the Covalent bond classification method. In the usual electron counting method, it is a two-electron ligand. Imidazole...
9 KB (1,042 words) - 15:24, 12 July 2025
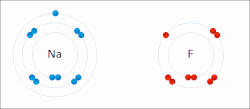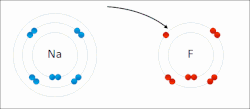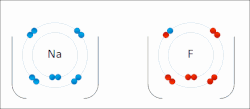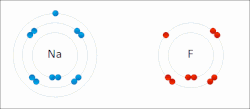
ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic...
18 KB (2,340 words) - 19:04, 15 June 2025
the metal. Based on the covalent bond classification method (from where LBN is derived), the equation for determining ligand bond number is as follows:...
5 KB (475 words) - 00:48, 28 June 2021
Chemical polarity (redirect from Polar covalent bond)
usually applied to covalent bonds, that is, bonds where the polarity is not complete. To determine the polarity of a covalent bond using numerical means...
24 KB (2,762 words) - 11:45, 25 May 2025
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as...
48 KB (5,593 words) - 15:34, 25 July 2025
pairs form a crystal structure with metallic bonding between them. Another example of a metal–metal covalent bond is the mercurous ion (Hg2+ 2). As chemistry...
24 KB (3,399 words) - 15:29, 5 July 2025
the transition metal); termed a "Z-type" ligand in Green's covalent bond classification method. The caveat originates from the simplifying use of electronegativity...
47 KB (12,003 words) - 15:23, 12 May 2025
Transition metal pyridine complexes (section Bonding)
Pyridine is classified as L ligand in the covalent bond classification method. In the usual electron counting method, it is a two-electron ligand. With respect...
22 KB (2,001 words) - 15:26, 12 July 2025
Organometallic chemistry (redirect from Metal carbon bonding)
letter kappa, κ. Chelating κ2-acetate is an example. The covalent bond classification method identifies three classes of ligands, X,L, and Z; which are...
31 KB (3,170 words) - 10:41, 18 July 2025
Electronegativity (category Chemical bonding)
estimate the bond energy, and the sign and magnitude of a bond's chemical polarity, which characterizes a bond along the continuous scale from covalent to ionic...
35 KB (4,352 words) - 13:00, 7 June 2025
ligands in these complexes are often labile. According to the Covalent bond classification method, nitriles are classified as L ligands, i.e., charge-neutral...
19 KB (1,995 words) - 10:50, 1 August 2025
bidentate. Unidentate nitrate is classified as X ligand in the Covalent bond classification method. With respect to HSAB theory, it is classified as hard. When...
11 KB (1,185 words) - 15:04, 11 April 2025
O bidentate amino carboxylates are "L-X" ligands in the Covalent bond classification method. With respect to HSAB theory, N,O bidentate amino carboxylate...
11 KB (1,220 words) - 22:15, 10 February 2025
Covalent adaptable networks (CANs) are a type of polymer material that closely resemble thermosetting polymers (thermosets). However, they are distinguished...
29 KB (3,354 words) - 22:37, 17 June 2025
complexes are used in medical imaging. According to the Covalent bond classification method, isocyanides are classified as L ligands, i.e., charge-neutral...
19 KB (1,822 words) - 17:49, 11 April 2025
Hydroxide is classified as an X ligand in the Covalent bond classification method. In the usual electron counting method, it is a one-electron ligand when terminal...
6 KB (663 words) - 15:47, 11 April 2025
Ligand (category Chemical bonding)
chemistry, ligands are classified according to the "CBC Method" for Covalent Bond Classification, as popularized by M. L. H. Green and "is based on the...
35 KB (3,363 words) - 09:12, 24 June 2025
C. Gibson. Green developed the covalent bond classification (CBC) method in 1995 to describe the ligands and bonding in coordination and organometallic...
15 KB (1,192 words) - 23:45, 23 May 2025
Thiolate is classified as an X ligand in the Covalent bond classification method. In the usual electron counting method, it is a one-electron ligand when terminal...
9 KB (1,014 words) - 22:59, 22 July 2025
are classified as L-X ligands in the Covalent bond classification method. In the usual electron counting method, they are three-electron ligands. With...
7 KB (811 words) - 13:05, 8 April 2025
viewed as simply somewhere along a continuum between idealized covalent bonding and ionic bonding. Lewis acids are diverse and the term is used loosely. Simplest...
22 KB (2,771 words) - 02:54, 24 June 2025
Metal ammine complex (section Structure and bonding)
Ammonia is classified as an L ligand in the Covalent bond classification method. In the usual electron counting method, it is a two-electron ligand. Ammonia...
15 KB (1,758 words) - 20:24, 21 June 2025
Periodic table (section Classification of elements)
thus forms a covalent H2 molecule, and boron forms a giant covalent structure based on icosahedral B12 clusters. In a metal, the bonding and antibonding...
251 KB (27,146 words) - 04:45, 30 July 2025
site of amides. Amides are thus L ligands according to the covalent bond classification method, i.e. charge-neutral 2e donors. With respect to HSAB theory...
11 KB (1,231 words) - 20:44, 14 July 2025
synthetic organic and organometallic contexts. According to the covalent bond classification method, vinylidenes are neutral L-type ligands donating two electrons...
15 KB (1,508 words) - 04:11, 15 July 2025
The simplest alkenyl ligand is vinyl. According to the covalent bond classification method, a terminal alkenyl is an anionic X-type ligand. Some alkenyl...
4 KB (420 words) - 22:30, 17 June 2025
that are classified as L-X ligand in the Covalent bond classification method. In the usual electron counting method, they are three-electron ligands. With...
12 KB (1,213 words) - 09:21, 23 June 2025