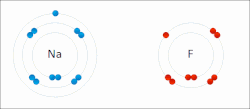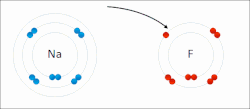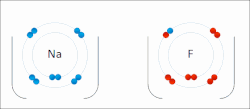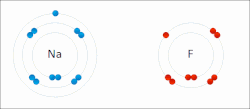
The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively...
35 KB (4,372 words) - 07:58, 25 May 2025
e Periodic table of electronegativity by Pauling scale → Atomic radius decreases → Ionization energy increases → Electronegativity increases → See also:...
13 KB (494 words) - 13:32, 15 May 2025
difference in electronegativity between the two atoms is less than 0.5 Polar bonds generally occur when the difference in electronegativity between the...
24 KB (2,763 words) - 11:45, 25 May 2025
Periodic trends (section Electronegativity)
known as electronegativity. It is a dimensionless quantity because it is only a tendency. The most commonly used scale to measure electronegativity was designed...
19 KB (2,130 words) - 01:00, 31 January 2025
extract with the electronegativity values of metals. Wulfsberg distinguishes: very electropositive metals with electronegativity values below 1.4 ...
18 KB (1,026 words) - 18:32, 28 May 2025
affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Two atoms with equal electronegativity will make...
29 KB (3,846 words) - 22:53, 13 April 2025
Periodic table (section Electronegativity)
scale cannot measure its electronegativity because it does not form covalent bonds with most elements. An element's electronegativity varies with the identity...
252 KB (27,179 words) - 14:30, 25 May 2025
strong bond differ due to the difference in electronegativity of the constituent elements. Electronegativity is the tendency for an atom of a given chemical...
40 KB (4,878 words) - 16:35, 25 May 2025
organized by other properties, such as atomic weight, density, and electronegativity. For more detailed information about the origins of element names...
2 KB (1,249 words) - 13:08, 20 March 2025
typically of higher electronegativity; in a bond between two atoms of the same element, the electrons are divided equally. Most electronegativity scales depend...
47 KB (12,011 words) - 15:23, 12 May 2025
metals and nonmetals depends on the electronegativity difference. Ionicity is possible when the electronegativity difference is high enough (e.g. Li3N...
19 KB (2,487 words) - 22:13, 25 May 2025
triangle, Norman's quantitative triangle) used electronegativity of compounds. Nowadays, electronegativity triangles are mostly used to rate the chemical...
3 KB (377 words) - 13:21, 4 May 2023
character – that is, a bond in which there is a large difference in electronegativity between the cation and anion, causing the bonding to be more polar...
18 KB (2,340 words) - 17:25, 5 February 2025
substituent electronegativities and it is for this work that Bent's rule takes its name. Bent's original paper considers the group electronegativity of the...
38 KB (4,285 words) - 19:40, 22 May 2025
less electronegative than fluoro groups—reduces the carboxylate oxygen charge density the most. This inversion of the traditional electronegativity–charge...
11 KB (1,381 words) - 15:57, 22 April 2025
tetrafluoride) are some of the most unreactive organic compounds. The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine...
15 KB (1,702 words) - 05:12, 25 May 2025
sulfide is a network solid made up of silver (electronegativity of 1.98) and sulfur (electronegativity of 2.58) where the bonds have low ionic character...
11 KB (970 words) - 07:28, 17 April 2025
Pauling's principle of electroneutrality (section Applications of the principle of electronegativity)
caesium fluoride has a polar covalent bond. The large difference in electronegativity gives a calculated covalent character of 9%. In the crystal (CsF has...
8 KB (1,159 words) - 04:27, 16 February 2025
chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number...
9 KB (1,050 words) - 02:14, 19 September 2024
polydimethylsiloxane. On the Pauling electronegativity scale, silicon has an electronegativity of 1.90 and oxygen 3.44. The electronegativity difference between the...
11 KB (1,124 words) - 12:41, 22 September 2024
picometres. Since fluorine is a relatively small atom with a large electronegativity, its covalent radius is difficult to evaluate. The covalent radius...
9 KB (1,286 words) - 08:12, 1 November 2023
outer shell. Corresponding to periodic trends, it is intermediate in electronegativity between chlorine and iodine (F: 3.98, Cl: 3.16, Br: 2.96, I: 2.66)...
67 KB (7,754 words) - 00:13, 25 May 2025
and ionic molecules.: 66 Here Pauling's electronegativity concept is particularly useful; the electronegativity difference between a pair of atoms will...
143 KB (14,181 words) - 05:41, 23 May 2025
electron affinities was used by Robert S. Mulliken to develop an electronegativity scale for atoms, equal to the average of the electrons affinity and...
22 KB (1,468 words) - 11:02, 24 April 2025
heat and electricity. Chemically, nonmetals have relatively high electronegativity or usually attract electrons in a chemical bond with another element...
175 KB (16,819 words) - 14:30, 22 May 2025
higher electronegativity values than metals noting that, in general, the higher an element's ionisation energy, electron affinity, and electronegativity, the...
34 KB (4,571 words) - 09:31, 19 November 2024
The noble elements gold and platinum also have a comparatively high electronegativity for a metallic element, thus allowing them to exist as single-metallic...
30 KB (2,955 words) - 18:01, 30 May 2025
technical standards Electroless nickel plating, a chemical technique Electronegativity, chemical tendency to attract electrons Engrailed (gene), a gene involved...
2 KB (333 words) - 18:21, 27 February 2025
Nightfall (band) (redirect from Electronegative (EP))
(1994) Eons Aura EP (1995) Athenian Echoes (1995) Lesbian Show (1997) Electronegative (1999) Diva Futura (1999) I Am Jesus (2003) Lyssa: Rural Gods and Astonishing...
15 KB (896 words) - 18:56, 18 May 2025
not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid...
23 KB (1,918 words) - 05:17, 26 May 2025