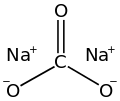
the enthalpy of neutralization (ΔnH) is the change in enthalpy that occurs when one equivalent of an acid and a base undergo a neutralization reaction...
3 KB (362 words) - 13:59, 22 April 2025
Enthalpy (/ˈɛnθəlpi/ ) is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics...
48 KB (6,108 words) - 20:45, 18 July 2025
Standard enthalpy of neutralization is the change in enthalpy that occurs when an acid and base undergo a neutralization reaction to form one mole of water...
16 KB (2,425 words) - 08:47, 25 November 2024
in water, neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The pH of the neutralized solution depends...
17 KB (2,128 words) - 01:06, 10 June 2025
of heat required to achieve a certain raise in the temperature of the calorimeter's contents. To determine the change in enthalpy in a neutralization...
2 KB (326 words) - 17:24, 21 November 2024
Sodium bicarbonate (redirect from Bicarbonate of soda)
Papuga A, Polańczyk A (6 December 2023). "Analysis of the latest guidelines for the neutralization of selected acids, including recommendations for emergency...
58 KB (5,469 words) - 17:19, 28 July 2025
Acid (redirect from List of Acids)
neutralization should result in a solution with pH 7.0, which is only the case with similar acid and base strengths during a reaction. Neutralization...
47 KB (5,960 words) - 01:33, 5 July 2025
others consider that it can be neutralized and poured down the drain with copious amounts of water. Improper neutralization can cause a fast decomposition...
20 KB (2,220 words) - 17:25, 6 August 2025
Sodium hydroxide (redirect from Manufacture of Sodium hydroxide by Nelson's process)
the lower enthalpy of formation of iron(III) oxide (−824.2 kJ/mol) compared to sodium hydroxide (−500 kJ/mol) and positive entropy change of the reaction...
55 KB (6,109 words) - 05:57, 6 August 2025
Enstrophy Enthalpy Enthalpy change of solution Enthalpy of fusion Enthalpy of neutralization Enthalpy of sublimation Enthalpy of vaporization Enthalpy–entropy...
20 KB (2,038 words) - 06:17, 14 June 2024
Thermometric titration (redirect from Enthalpy titration)
interpretation on the part of the analyst as to their location. Enthalpy change is arguably the most fundamental and universal property of chemical reactions...
35 KB (4,524 words) - 14:08, 6 August 2025
Uranium acid mine drainage (category Environmental impact of mining)
Gibbs free energy of formation values to also be spontaneous. Table 1. The enthalpy of formation (from oxide to mineral), enthalpy of formation (from individual...
12 KB (1,359 words) - 11:54, 27 February 2025
Sulfuric acid (redirect from Oil of vitriol)
The above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply fluoroantimonic...
65 KB (7,192 words) - 21:14, 20 July 2025
at 37 °C. The standard enthalpy of formation of rubidium fluoride is ΔfH0298 = −552.2 kJ mol−1, the standard free enthalpy of formation ΔG0298 = −520...
7 KB (490 words) - 22:47, 10 March 2025
Sodium hypochlorite (redirect from Chloride of soda)
hypochlorite is also used to neutralize any accidental releases of the nerve agent in the toxic areas. Lesser concentrations of sodium hypochlorite are used...
55 KB (5,750 words) - 07:20, 19 July 2025
Sodium carbonate (category Types of ash)
processing and tanning of animal hides. The integral enthalpy of solution of sodium carbonate is −26.7 kJ/mol . The Mohs hardness of sodium carbonate monohydrate...
32 KB (3,055 words) - 19:41, 29 July 2025
Sodium chloride (redirect from Muriate of soda)
from the original on 19 September 2018. Retrieved 18 September 2018. Neutralization of a strong acid and a strong base gives a neutral salt. Burgess, J (1978)...
36 KB (3,739 words) - 05:35, 25 July 2025
Mercury sulfide (redirect from Cinnabar of antimony)
storage. With a band gap of 2.1 eV and its stability, it is possible to be used as photoelectrochemical cell. Neutralization with sulfur has been suggested...
7 KB (552 words) - 04:36, 21 June 2025
Sodium nitrate (redirect from Nitrate of soda)
energy recovery, owing to its relatively high melting enthalpy of 178 J/g. Examples of the applications of sodium nitrate used for thermal energy storage include...
19 KB (1,740 words) - 02:33, 23 June 2025
Americium (redirect from History of americium)
G.; Petereson, J. R. (1987). "The enthalpy of solution of 243Am metal and the standard enthalpy of formation of Am3+(aq)". Thermochimica Acta. 116:...
77 KB (9,341 words) - 13:31, 26 July 2025
ultra-alkaline pegmatites. Sodium oxalate can be prepared through the neutralization of oxalic acid with sodium hydroxide (NaOH) in a 1:2 acid-to-base molar...
7 KB (592 words) - 04:47, 15 July 2025
in a fluid where there is an enthalpy change, although reaction kinetics can play a role in determining the sharpness of the endpoint. Thermometric titrimetry...
8 KB (1,118 words) - 08:14, 21 May 2024
Magnesium hydroxide (redirect from Milk of Magnesia)
by simple neutralization, in which the hydroxide ions from the Mg(OH)2 combine with acidic H+ ions (or hydronium ions) produced in the form of hydrochloric...
24 KB (2,400 words) - 22:58, 23 July 2025
Carbon dioxide (redirect from Biological roles of carbon dioxide)
widespread uses in industry because they can be used to neutralize waste acid streams. Around 230 Mt of CO2 are used each year, mostly in the fertiliser industry...
112 KB (12,779 words) - 13:18, 15 July 2025
Caesium (redirect from Compounds of caesium)
Griffiths, T. T. (2006). "Determination of the temperature and enthalpy of the solid–solid phase transition of caesium nitrate by differential scanning...
91 KB (9,838 words) - 12:27, 9 August 2025
Potassium hydroxide (section Manufacture of soft soaps)
factories. Many potassium salts are prepared by neutralization reactions involving KOH. The potassium salts of carbonate, cyanide, permanganate, phosphate...
21 KB (2,066 words) - 14:15, 29 July 2025
Ammonia (redirect from Biosynthesis of ammonia)
ionising powers reflecting its high ε of 22 at −35 °C (−31 °F). Liquid ammonia has a very high standard enthalpy change of vapourization (23.5 kJ/mol; for comparison...
141 KB (15,151 words) - 06:53, 21 July 2025
orthophosphate or simply sodium phosphate. Trisodium phosphate is produced by neutralization of phosphoric acid using sodium carbonate, which produces disodium hydrogen...
12 KB (951 words) - 22:26, 29 May 2025
used for both industrial and consumer products. The triethanolamine neutralizes fatty acids, adjusts and buffers the pH, and solubilizes oils and other...
14 KB (1,267 words) - 10:34, 7 June 2025
Curium (redirect from History of curium)
of Inorganic and Nuclear Chemistry. 29 (11): 2753. doi:10.1016/0022-1902(67)80014-9. Fuger, J.; Haire, R.; Peterson, J. (1993). "Molar enthalpies of formation...
66 KB (7,688 words) - 10:15, 8 August 2025