Look up exergonic in Wiktionary, the free dictionary. An exergonic process is one which there is a positive flow of energy from the system to the surroundings...
3 KB (414 words) - 09:09, 8 May 2025
In chemical thermodynamics, an exergonic reaction is a chemical reaction where the change in the free energy is negative (there is a net release of free...
3 KB (297 words) - 15:14, 11 October 2024
spontaneous process at a certain temperature, the products have a lower Gibbs free energy G = H – TS than the reactants (an exergonic process), even if...
9 KB (941 words) - 16:28, 17 July 2025
Endergonic reaction (category Thermodynamic processes)
they are either pulled or pushed by an exergonic (stability increasing, negative change in free energy) process. Of course, in all cases the net reaction...
7 KB (938 words) - 19:07, 3 July 2025
Differential scanning calorimetry Endergonic Endergonic reaction Exergonic Exergonic reaction Endothermic reaction "Gate for the Greek language" on-line...
10 KB (1,020 words) - 21:03, 3 June 2025
The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton...
34 KB (4,081 words) - 05:42, 19 July 2025
Exothermic reactions usually release heat. The term is often confused with exergonic reaction, which IUPAC defines as "... a reaction for which the overall...
5 KB (552 words) - 07:35, 19 March 2024
This is reflected in a negative ΔG, and the reaction is called an exergonic process.[citation needed] If two chemical reactions are coupled, then an otherwise...
33 KB (4,550 words) - 21:59, 19 June 2025
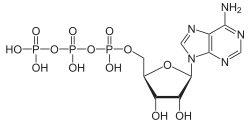
acidosis. Hydrolysis of the terminal phosphoanhydridic bond is a highly exergonic process. The amount of released energy depends on the conditions in a particular...
10 KB (1,228 words) - 08:44, 9 February 2025
Nitrogen cycle (section Processes)
conjugated base: NH+4 + NO−2 → N2 + 2 H2O (ΔG° = −357 kJ⋅mol−1). This an exergonic process (here also an exothermic reaction) releasing energy, as indicated...
66 KB (6,980 words) - 21:54, 22 May 2025
exergonic and endergonic reactions, see the separate articles: Endergonic reaction Exergonic reaction Exergonic process Endergonic Exothermic process...
237 bytes (54 words) - 20:02, 8 November 2017
oxidative phosphorylation, which makes tunneling of the cation an exergonic process. Normal, mild calcium influx from cytosol into the mitochondrial matrix...
172 KB (18,911 words) - 08:14, 18 July 2025
and hydrogen (protons), is an exergonic process – it releases energy, whereas the synthesis of ATP is an endergonic process, which requires an input of...
102 KB (11,252 words) - 20:48, 27 June 2025
oligosaccharide and polysaccharide. dispersive replication dissimilatory process Any exergonic process of microbial catabolism by which redox-active chemical species...
340 KB (31,622 words) - 15:06, 3 July 2025
Water–gas shift reaction (redirect from Gas shift process)
WGSR is exergonic, with the following thermodynamic parameters at room temperature (298 K) In aqueous solution, the reaction is less exergonic. In the...
22 KB (2,710 words) - 12:35, 26 June 2025
mechanism proceeds as a concerted, but asynchronous step and is an exergonic process. The mechanism for this transformation is formally a Claisen rearrangement...
13 KB (1,364 words) - 13:52, 19 July 2025
Thermochemistry (section Processes)
the concepts of exothermic and endothermic reactions are generalized to exergonic reactions and endergonic reactions. Thermochemistry rests on two generalizations...
7 KB (788 words) - 18:00, 26 June 2025
Phillips catalyst (category Industrial processes)
industrially by the polymerization of ethylene: n C2H4 → (C2H4)n Although exergonic (i.e., thermodynamically favorable), the reaction requires catalysts....
3 KB (293 words) - 18:33, 10 December 2024
solvation energy is positive, then the solvation process is endergonic; otherwise, it is exergonic. For instance, water warms when treated with CaCl2...
3 KB (342 words) - 19:54, 15 February 2025
characteristics of a chemical reaction, such as whether the reaction is exergonic or endergonic. Additionally, the properties of a product can make it easier...
9 KB (1,082 words) - 14:30, 20 June 2025
To drive the reaction forward, the reaction is coupled to a strongly exergonic hydrolysis reaction: the enzyme inorganic pyrophosphatase cleaves the...
6 KB (683 words) - 20:38, 26 June 2025
dehydrating them. As a consequence, the hydrolysis of these bonds is exergonic under physiological conditions, releasing Gibbs free energy.[citation...
6 KB (692 words) - 17:18, 29 December 2024
less energy than the reactants. A reaction is said to be exothermic or exergonic if the final state is lower on the energy scale than the initial state;...
69 KB (8,281 words) - 00:58, 28 July 2025
[citation needed] The negative ΔG indicates that the reaction is exothermic (exergonic) and can occur spontaneously. The potential of NADH and FADH2 is converted...
31 KB (3,357 words) - 05:05, 1 July 2025
3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation with Δ(ΔfH) ca. −25 kcal/mol (−100 kJ/mol)...
24 KB (2,568 words) - 10:05, 25 June 2025
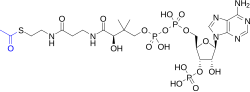
which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol). CoA is acetylated to acetyl-CoA by the breakdown of carbohydrates...
29 KB (2,729 words) - 21:28, 8 July 2025
the organism for other purposes, such as breaking chemical bonds. An exergonic reaction is a spontaneous chemical reaction that releases energy. It is...
27 KB (3,539 words) - 17:19, 17 July 2025
catalyst, and a suitable dye (sensitizer, or fluorophor). This creates an exergonic reaction. The chemicals inside the plastic tube are a mixture of the dye...
28 KB (3,125 words) - 08:58, 22 July 2025
is coupled to the hydrolysis of ATP or GTP, effectively making the process exergonic. For example, the pathway leading from pyruvate to glucose-6-phosphate...
33 KB (3,700 words) - 02:48, 28 June 2025
while the formation of proteins from amino acids is an anabolic process. Exergonic reactions are energy-releasing reactions and are generally catabolic...
47 KB (6,228 words) - 05:03, 21 June 2025