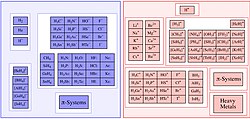
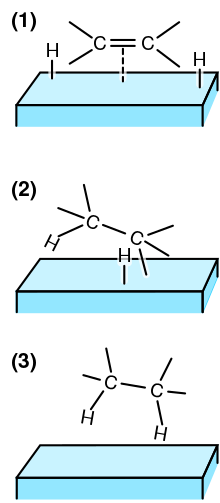
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form...
23 KB (2,669 words) - 15:05, 26 May 2025
Tris(pentafluorophenyl)borane is a key reagent leading to the concept of frustrated Lewis pairs. The combination of BCF and bulky basic phosphines, such as tricyclohexylphosphine...
11 KB (1,100 words) - 11:53, 22 April 2025
an electron pair or Lewis pair consists of two electrons that occupy the same molecular orbital but have opposite spins. Gilbert N. Lewis introduced the...
3 KB (314 words) - 05:55, 25 May 2025
electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is...
22 KB (2,774 words) - 08:02, 25 May 2025
Conjugate (acid-base theory) (redirect from Conjugate acid-base pair)
the base and the proton is shown by an arrow that starts on an electron pair from the base and ends at the hydrogen ion (proton) that will be transferred:...
13 KB (1,310 words) - 14:02, 17 May 2025
thionylamide, HNSO, can be made that way at low temperature. A frustrated Lewis pair, such as tris(tert-butyl) phosphine and tris(pentafluorophenyl)borane...
5 KB (444 words) - 15:22, 13 December 2024
017 S. Brand, J. Pahl, H. Elsen, and S. Harder, "Frustrated Lewis pair chemistry with magnesium Lewis acids", European J. Inorg. Chem., 2017, 4187-4195...
7 KB (972 words) - 06:42, 20 May 2025
Acid–base reaction (section Lewis definition)
substances, the Lewis definition defines a base (referred to as a Lewis base) to be a compound that can donate an electron pair, and an acid (a Lewis acid) to...
36 KB (4,977 words) - 16:27, 29 May 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
24 KB (2,745 words) - 02:45, 19 May 2025
a compound that can give an electron pair to a Lewis acid, a compound that can accept an electron pair. Lewis's proposal explains the Brønsted–Lowry classification...
16 KB (1,949 words) - 16:30, 29 May 2025
acidity to the weakness of proton acceptors (and electron pair donors) (Brønsted or Lewis bases) in solution. Because of this, the protons in fluoroantimonic...
13 KB (1,515 words) - 08:12, 15 April 2025
Acid strength (section Conjugate acid/base pair)
Lewis acids toward a series of bases, versus other Lewis acids, can be illustrated by C-B plots. It has been shown that to define the order of Lewis acid...
19 KB (2,474 words) - 21:52, 17 April 2025
Base (chemistry) (section Lewis bases)
In the Lewis theory, a base is an electron pair donor which can share a pair of electrons with an electron acceptor which is described as a Lewis acid....
26 KB (3,045 words) - 01:37, 24 May 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
10 KB (1,203 words) - 20:01, 24 May 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
9 KB (1,368 words) - 08:10, 2 June 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
22 KB (2,336 words) - 05:58, 25 May 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
2 KB (248 words) - 02:34, 22 April 2025
test solution "p" and the reference solution "q"; these letters are often paired with e4 then e5. Some literature sources suggest that "pH" stands for the...
53 KB (6,540 words) - 00:39, 24 May 2025
"Phosphazenyl Phosphines: The Most Electron-Rich Uncharged Phosphorus Brønsted and Lewis Bases". Angewandte Chemie International Edition. 58 (30): 10335–10339. doi:10...
12 KB (1,181 words) - 13:14, 4 December 2024
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
19 KB (2,147 words) - 06:25, 26 December 2024
homogeneous catalysis and, with B(C6F5)3, comprises the classic frustrated Lewis pair. 2,6-Di-tert-butylphenol is used industrially as UV stabilizers...
11 KB (993 words) - 10:27, 15 March 2025
Acid (section Lewis acids)
Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry...
47 KB (5,960 words) - 15:34, 29 May 2025
former party (Spanish: Frente de Liberación Popular) Flurbiprofen Frustrated Lewis pair Lugano–Ponte Tresa railway (Italian: Ferrovia Lugano–Ponte Tresa)...
1 KB (142 words) - 23:43, 12 May 2024
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
9 KB (778 words) - 14:36, 20 November 2024
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
19 KB (1,671 words) - 05:48, 19 April 2025
Self-ionization of water Titration Lewis acid catalysis Frustrated Lewis pair Chiral Lewis acid ECW model Acid types Brønsted–Lowry Lewis Mineral Organic Oxide Strong...
8 KB (949 words) - 17:08, 7 March 2025
or acts as an acceptor of hydroxide ions effectively functioning as a Lewis acid. Acidic oxides will typically have a low pKa and may be inorganic or...
5 KB (592 words) - 21:19, 19 May 2025
that cannot form an adduct because of steric hindrance are called frustrated Lewis pairs. Adducts are not necessarily molecular in nature. A good example...
4 KB (529 words) - 07:04, 16 January 2025
test tube or in the extracellular fluid. Buffers typically consist of a pair of compounds in solution, one of which is a weak acid and the other a weak...
21 KB (2,428 words) - 15:03, 20 November 2024
based on the phosphine-borane, compound 1, which has been called a frustrated Lewis pair. It reversibly accepts dihydrogen at relatively low temperatures...
39 KB (4,108 words) - 14:29, 26 May 2025