In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive...
10 KB (1,113 words) - 22:59, 29 March 2025
In organic chemistry, α-keto halogenation is a special type of halogenation. The reaction may be carried out under either acidic or basic conditions in...
7 KB (812 words) - 22:33, 23 May 2025
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics...
8 KB (853 words) - 03:10, 30 April 2025
Darzens halogenation is the chemical synthesis of alkyl halides from alcohols via the treatment upon reflux of a large excess of thionyl chloride or thionyl...
3 KB (281 words) - 05:58, 23 April 2024
In organic chemistry, an electrophilic aromatic halogenation is a type of electrophilic aromatic substitution. This organic reaction is typical of aromatic...
7 KB (828 words) - 06:48, 14 December 2023
Friedel–Crafts reaction (redirect from Friedel-Crafts halogenation)
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts...
22 KB (2,159 words) - 16:16, 24 May 2025
Ethanol (section Halogenation)
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound with the chemical formula CH3CH2OH. It is...
109 KB (10,861 words) - 00:50, 5 June 2025
Haloform reaction (category Halogenation reactions)
haloform (CHX3, where X is a halogen) is produced by the exhaustive halogenation of an acetyl group (R−C(=O)CH3, where R can be either a hydrogen atom...
11 KB (1,324 words) - 20:06, 4 October 2024
The Hell–Volhard–Zelinsky halogenation reaction is a chemical transformation that transforms an alkyl carboxylic acid to the α-bromo derivative. It is...
6 KB (540 words) - 06:22, 2 June 2025
Ether (section Alpha-halogenation)
In organic chemistry, ethers are a class of compounds that contain an ether group, a single oxygen atom bonded to two separate carbon atoms, each part...
19 KB (1,841 words) - 14:31, 5 May 2025
Hydrocarbon (section Halogenation)
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon.: 620 Hydrocarbons are examples of group 14 hydrides...
26 KB (2,765 words) - 19:19, 28 May 2025
Buckminsterfullerene (section Halogenation)
Buckminsterfullerene is a type of fullerene with the formula C 60. It has a cage-like fused-ring structure (truncated icosahedron) made of twenty hexagons...
47 KB (4,708 words) - 15:41, 4 June 2025
Triphosgene is stable up to 200 °C. Triphosgene is used in a variety of halogenation reactions. This compound is commercially available. It is prepared by...
8 KB (648 words) - 17:04, 18 April 2025
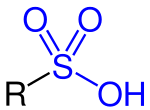
Sulfonic acid (section Halogenation)
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula R−S(=O)2−OH...
16 KB (1,896 words) - 05:45, 7 April 2025
Fullerene chemistry (section Halogenation)
Fullerene chemistry is a field of organic chemistry devoted to the chemical properties of fullerenes. Research in this field is driven by the need to functionalize...
41 KB (4,408 words) - 16:52, 24 May 2025
Sandmeyer reaction (section Halogenation)
through which one can perform unique transformations on benzene, such as halogenation, cyanation, trifluoromethylation, and hydroxylation. The reaction was...
16 KB (1,738 words) - 11:35, 22 April 2025
Sulfur (section Halogenation)
Sulfur (American spelling and the preferred IUPAC name) or sulphur (Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16...
98 KB (10,893 words) - 14:32, 6 June 2025
Enamine (section Halogenation)
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates...
17 KB (1,832 words) - 16:29, 5 June 2025
Fluorochemical industry (section Halogenation)
The global market for chemicals from fluorine was about US$16 billion per year as of 2006. The industry was predicted to reach 2.6 million metric tons...
37 KB (3,684 words) - 03:17, 9 June 2025
Organoboron chemistry (section Halogenation)
the boron atom may convert to halide, but disiamylborane permits only halogenation of the hydroborated olefin: Treatment of an alkenylborane with iodine...
32 KB (3,220 words) - 09:34, 25 May 2025
Diphenyl disulfide (section Halogenation)
Diphenyl disulfide is the chemical compound with the formula (C6H5S)2. This colorless crystalline material is often abbreviated Ph2S2. It is one of the...
6 KB (472 words) - 12:57, 26 May 2025
electrons, play a large role in most reactions of alkanes. Free radical halogenation reactions occur with halogens, leading to the production of haloalkanes...
63 KB (7,004 words) - 08:31, 4 June 2025
reactions of this type that take place are aromatic nitration, aromatic halogenation, aromatic sulfonation and acylation and alkylating Friedel-Crafts reactions...
2 KB (244 words) - 01:58, 6 April 2023
particularly common α-substitution reaction in the laboratory is the halogenation of aldehydes and ketones at their α positions by reaction Cl2, Br2 or...
7 KB (982 words) - 20:11, 11 November 2024
Ethylene (section Halogenation and hydrohalogenation)
ethylene include in order of scale: 1) polymerization, 2) oxidation, 3) halogenation and hydrohalogenation, 4) alkylation, 5) hydration, 6) oligomerization...
36 KB (3,225 words) - 20:40, 8 June 2025
organolithium reagents: RLi + CO2 → RCO−2Li+ RCO−2Li+ + HCl → RCO2H + LiCl Halogenation followed by hydrolysis of methyl ketones in the haloform reaction Base-catalyzed...
27 KB (2,706 words) - 03:04, 26 May 2025
Hydantoin (section Halogenation)
Hydantoin, or glycolylurea, is a heterocyclic organic compound with the formula CH2C(O)NHC(O)NH. It is a colorless solid that arises from the reaction...
9 KB (822 words) - 22:56, 19 March 2025
isomerism is taken into account. Bromophenols are produced by electrophilic halogenation of phenol with bromine. There is a total of 19 bromophenols, corresponding...
3 KB (369 words) - 03:12, 9 June 2025
strong oxidizing agent used as a source of electrophilic bromine in halogenation reactions. The analogous quinoline compound behaves similarly. Pyridinium...
3 KB (256 words) - 12:48, 16 May 2025
Carbon nanotube chemistry involves chemical reactions, which are used to modify the properties of carbon nanotubes (CNTs). CNTs can be functionalized to...
34 KB (3,689 words) - 16:00, 23 May 2025