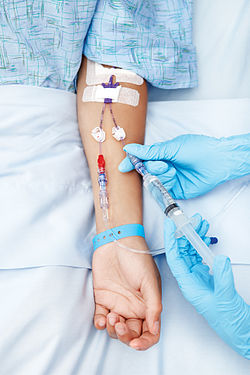
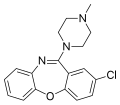
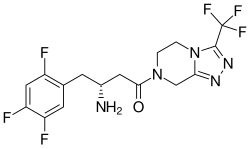
A package insert is a document included in the package of a medication that provides information about that drug and its use. For prescription medications...
11 KB (1,313 words) - 21:27, 29 May 2025
the package insert or consult a health professional for the amount of drops to use and the duration of treatment. Prior to using the medication, refer...
18 KB (2,082 words) - 05:40, 19 July 2025
prescription medications, the insert is technical, and provides information for medical professionals about how to prescribe the drug. Package inserts for prescription...
21 KB (2,185 words) - 20:39, 29 May 2025
(S)-methamphetamine, or metamfetamine (INN), respectively. The medication package insert for Desoxyn lists the chemical name (S)-N,α-dimethylbenzeneethanamine...
170 KB (16,593 words) - 00:04, 29 July 2025
Intravenous therapy (redirect from Intravenous medication)
(abbreviated as IV therapy) is a medical process that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of...
60 KB (7,182 words) - 17:24, 27 July 2025
take clopidogrel, cardiovascular risk is elevated, leading to medication package insert updates by regulators. In patients with type 2 diabetes, haptoglobin...
65 KB (6,724 words) - 12:51, 18 July 2025
early as the Han dynasty. The medication is approved in China as a Chinese patent medicine. As a result, the package insert includes a list of herbs, but...
18 KB (1,890 words) - 01:18, 13 July 2025
policy of the United States Drug policy of the United Kingdom Medication package insert Auxiliary label F. Janssen, Wallace (June 1981). "The Story of...
17 KB (1,766 words) - 20:39, 29 May 2025
requires all antidepressants to include that boxed warning on medication package inserts. The safety and effectiveness of Naltrexone/bupropion in children...
21 KB (1,606 words) - 21:36, 29 May 2025
colloquially) is a type of warning that appears near the beginning of the package insert for certain prescription drugs, so called because the U.S. Food and...
18 KB (1,875 words) - 21:20, 11 July 2025
Genuine in the slides and the medication package insert. A boxed warning is present at the beginning of the Chinese package insert, describing a risk of "decrease...
18 KB (2,078 words) - 14:38, 13 April 2025
(1): 213–221. doi:10.1016/0378-4347(91)80083-O. PMID 1860915. "ADASUVE Package Insert" (PDF). Galen US Inc. "Clozapine and loxapine for schizophrenia". Drug...
18 KB (1,270 words) - 06:24, 19 July 2025
Betahistine (redirect from Serc (medication))
Betahistine may also cause several digestive-related adverse effects. The package insert for Serc, a trade name for betahistine, states that patients may experience...
17 KB (1,791 words) - 02:43, 20 July 2025
usually provided with it, either in monographs, package inserts (accompanying prescribed medications), or in warning labels (for OTC drugs). CDI/MDI might...
6 KB (688 words) - 02:21, 25 May 2025
discontinued, is a compilation of manufacturers' prescribing information (package insert) on prescription drugs, updated regularly and published by ConnectiveRx...
5 KB (538 words) - 14:03, 25 December 2024
injections into the deltoid muscle of the upper arm. Statements in medication package inserts listing the frequency of side effects describe how often the effect...
324 KB (32,744 words) - 23:32, 29 July 2025
Lisinopril is a medication belonging to the drug class of angiotensin-converting enzyme (ACE) inhibitors and is used to treat hypertension (high blood...
22 KB (1,885 words) - 03:45, 14 May 2025
Metformin (section Combination with other medications)
under the brand name Glucophage, among others, is the main first-line medication for the treatment of type 2 diabetes, particularly in people who are overweight...
158 KB (15,092 words) - 14:14, 22 July 2025
treated with sitagliptin and other DPP-4 inhibitors, and the US FDA package insert carries a warning to this effect, although the causal link between sitagliptin...
22 KB (1,752 words) - 22:11, 29 May 2025
Prescription drug (redirect from Prescription medication)
A prescription drug (also prescription medication, prescription medicine or prescription-only medication) is a pharmaceutical drug that is permitted to...
32 KB (3,378 words) - 13:45, 1 June 2025
Humulin was the first medication produced using modern genetic engineering techniques in which actual human DNA is inserted into a host cell (E. coli...
108 KB (11,453 words) - 07:28, 13 July 2025
Sucralfate (category Equine medications)
Sucralfate, sold under various brand names, is a medication used to treat stomach ulcers, gastroesophageal reflux disease (GERD), radiation proctitis,...
20 KB (1,901 words) - 18:42, 16 July 2025
1136/bmjoq-2023-002382. PMC 11086455. PMID 38719523. "Piperacillin and Tazobactam: Package Insert". El-Haffaf I, Caissy JA, Marsot A (July 2021). "Piperacillin-Tazobactam...
12 KB (1,023 words) - 17:54, 17 July 2025
Duloxetine, sold under the brand name Cymbalta among others, is a medication used to treat major depressive disorder, generalized anxiety disorder, obsessive–compulsive...
76 KB (7,456 words) - 09:47, 29 July 2025
Institutional Review Board Drug susceptibility to abuse Proposed labeling (package insert) and directions for use Exceptions to this process include voter driven...
15 KB (1,533 words) - 17:36, 17 July 2025
Mechanisms and Management". Medscape. Retrieved 2 April 2014. "Lotensin package insert" (PDF). U.S. Food and Drug Administration (FDA). 2011. Retrieved 9 December...
9 KB (479 words) - 02:25, 21 December 2024
Notice by the Food and Drug Administration on 11/20/2015. Liptruzet [package insert]. Whitehouse Station, NJ: Merck & Co.; 2013 Clinical trial number NCT00418834...
5 KB (397 words) - 19:49, 20 December 2023
Omeprazole (category Dog medications)
Omeprazole, sold under the brand names Prilosec and Losec among others, is a medication used in the treatment of gastroesophageal reflux disease (GERD), peptic...
45 KB (4,069 words) - 05:50, 28 July 2025
asthenia, fatigue, and malaise in the Adverse Reactions section of the package insert. Archived from the original on August 23, 2009. Retrieved 2008-07-21...
11 KB (717 words) - 21:24, 15 July 2025
List of adverse effects of aripiprazole (category Medication side effects)
1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212. "ABILIFY (aripiprazole) [package insert]" (PDF). Otsuka Pharmaceutical Co, Ltd. Retrieved 18 October 2012. Felin...
9 KB (723 words) - 17:47, 16 July 2025