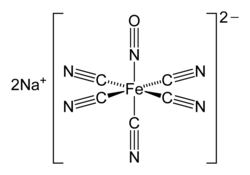
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way for historical...
15 KB (1,758 words) - 18:29, 10 February 2025
Some of the simplest members of such complexes are described in metal aquo complexes, metal ammine complexes, Examples: [Co(EDTA)]−, [Co(NH3)6]3+, [Fe(C2O4)3]3-...
57 KB (5,547 words) - 11:29, 21 May 2025
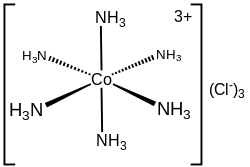
Hexaamminecobalt(III) chloride (category Ammine complexes)
of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion. [Co(NH3)6]3+...
8 KB (665 words) - 21:00, 6 August 2024
Hexaammineplatinum(IV) chloride (category Ammine complexes)
[Pt(NH3)6]Cl4. It is the chloride salt of the metal ammine complex [Pt(NH3)6]4+. The cation features six ammonia (called ammines in coordination chemistry) ligands...
3 KB (180 words) - 12:16, 13 January 2025
2 H2O Phosphines are L-type ligands. Unlike most metal ammine complexes, metal phosphine complexes tend to be lipophilic, displaying good solubility in...
14 KB (1,418 words) - 17:55, 22 June 2024
[LnMNO] + OH− The reaction is reversible in some cases. In some metal-ammine complexes, the ammonia ligand can be oxidized to nitrosyl: H2O + [Ru(terpy)(bipy)(NH3)]+...
18 KB (2,128 words) - 16:41, 4 April 2025
cationic metal ammine complexes such as [Pt(NH3)6]4+ spontaneously convert to the amido derivative: [Pt(NH3)6]4+ ↔ [Pt(NH3)5(NH2)]3+ + H+ Transition metal amides...
7 KB (777 words) - 10:45, 29 June 2024
Hexaamminenickel chloride (category Ammine complexes)
[Ni(NH3)6]Cl2. It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands...
4 KB (256 words) - 09:21, 6 January 2025
complex. For example, water exchange in [Al(H2O)5OH]2+ is 20000 times faster than in [Al(H2O)6]3+. Hydration number Ligand field theory Metal ammine complex...
15 KB (1,560 words) - 10:27, 14 May 2025
prominent examples of metal-ammine-chlorides. As indicated in the table below, many hydrates of metal chlorides are molecular complexes. These compounds are...
61 KB (3,492 words) - 19:29, 25 March 2025
(disambiguation) Amino (disambiguation) Anime, Japanese animation Metal ammine complex This disambiguation page lists articles associated with the title...
419 bytes (87 words) - 16:31, 31 October 2022
Azanide (section Alkali metal derivatives)
Transition metal complexes of the amido ligand are often produced by salt metathesis reaction or by deprotonation of metal ammine complexes. Bergstrom...
4 KB (277 words) - 11:05, 22 October 2024
Pentaamine(dinitrogen)ruthenium(II) chloride (category Ammine complexes)
the pi backbonding, the donation of metal d-electrons into the N2 π* orbitals. The related metal ammine complex [Os(NH3)5(N2)]2+ is also known. The dinitrogen...
5 KB (466 words) - 16:05, 7 April 2024
In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite (−NO2) ligands...
9 KB (1,029 words) - 07:15, 5 March 2025
like most ions, have integer oxidation states. For example, ruthenium ammine complexes are typically +2 or +3. The fact that the oxidation states are half-integer...
4 KB (464 words) - 15:15, 18 December 2023
Schweizer's reagent (category Ammine complexes)
Schweizer's reagent is a metal ammine complex with the formula [Cu(NH3)4(H2O)2](OH)2. This deep-blue compound is used in purifying cellulose. This salt...
7 KB (734 words) - 16:45, 20 November 2024
sulfate. Well characterized xamples are found with cobalt(III) ammines since these complexes are exchange inert. Monodentate sulfate is found in [Co(tren)(NH3)(SO4)]+...
8 KB (826 words) - 02:05, 3 January 2025
Rhodium(III) chloride (section Coordination complexes)
chloride, [RhCl(NH3)5]Cl2. As for other metal-ammine complexes, the term "ammine" refers to ammonia bound to a metal ion as a ligand. Zinc reduction of this...
19 KB (1,917 words) - 12:33, 22 May 2025
A transition metal imidazole complex is a coordination complex that has one or more imidazole ligands. Complexes of imidazole itself are of little practical...
9 KB (1,011 words) - 18:37, 10 February 2025
general method for the determination of stability constants of metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly...
60 KB (7,556 words) - 10:18, 14 May 2025
Magnus's green salt (category Ammine complexes)
"Vauquelin’s salt". Magnus's green salt was one of the first examples of a metal ammine complex. Atoji, Masao; Richardson, James W.; Rundle, R. E. (June 1957). "Pt(NH3)4PtCl41"...
6 KB (538 words) - 03:55, 3 March 2025
forming metal ammine complexes. For historical reasons, ammonia is named ammine in the nomenclature of coordination compounds. One notable ammine complex is...
139 KB (15,028 words) - 12:45, 23 May 2025
coordination chemistry and organometallic chemistry, transition metal imido complexes is a coordination compound containing an imido ligand. Imido ligands...
6 KB (514 words) - 16:47, 30 September 2023
Ligand (section Metal–ligand multiple bond)
in the cobalt ammine chlorides and to explain many of the previously inexplicable isomers. He resolved the first coordination complex called hexol into...
35 KB (3,307 words) - 14:02, 22 November 2024
which features a pair of S-bonded thiosulfate ligands. Simple aquo and ammine complexes are also known. Three binding modes are common: monodentate (κ1-),...
6 KB (714 words) - 02:38, 17 December 2024
Reinecke's salt (category Ammine complexes)
boiling water, acetone, and ethanol. It can be classified as a metal isothiocyanate complex. The chromium atom is surrounded by six nitrogen atoms in an...
5 KB (264 words) - 13:21, 24 April 2025
directly to the metal. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes, ion pairing...
7 KB (723 words) - 15:05, 24 May 2025
reconciled the low reactivity of the ammonia molecules present in metal ammine complexes by theorizing that the ammonia molecules were chemically linked...
13 KB (1,241 words) - 07:42, 14 April 2025
Chloropentamminecobalt chloride (category Ammine complexes)
coordination complexes feature metal atoms of octahedral and tetrahedral shapes, with ammonia and other ligands attached individually to the metal. Werner's...
6 KB (572 words) - 20:36, 7 May 2025
Platinosis (category Toxic effects of metals)
and ammine and nitro complexes such as [Pt(NH3)4]Cl2, K2[Pt(NO2)4] and platinum nitrate are not considered to be allergenic; neither is the metal. Heavy...
3 KB (368 words) - 02:48, 13 January 2024