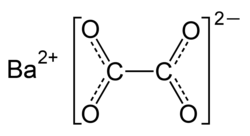
An oxalate nitrate is a chemical compound or salt that contains oxalate and nitrate anions (NO3− and C2O42-). These are mixed anion compounds. Some have...
39 KB (2,151 words) - 13:30, 22 May 2025
co-precipitation using dysprosium nitrate hexahydrate, oxalic acid dihydrate, and ethylene glycol. Several dysprosium oxalate metal organic frameworks (MOFs)...
8 KB (598 words) - 01:32, 24 June 2025
140 degrees Celsius, shock or friction. Silver oxalate is produced by the reaction between silver nitrate and oxalic acid. Dioxane tetraketone John Rumble...
3 KB (125 words) - 17:35, 24 March 2025
anions. It reacts with oxalate to give uranyl oxalate. Treatment with hydrochloric acid gives uranyl chloride. Uranyl nitrate is an oxidizing and highly...
8 KB (687 words) - 16:09, 24 January 2025
Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly...
29 KB (2,571 words) - 11:24, 4 April 2025
satisfied without commonly used oxidizers as nitrates, chlorates and perchlorates. Though largely stable, barium oxalate can be reactive with strong acids. A...
3 KB (204 words) - 01:40, 26 December 2024
Gadolinium oxalate is the oxalate of gadolinium, with the chemical formula Gd2(C2O4)3. Its hydrate can be prepared by the reaction of gadolinium nitrate and...
4 KB (173 words) - 00:24, 18 June 2025
Lanthanum oxalate is an inorganic compound, a salt of lanthanum metal and oxalic acid with the chemical formula La 2(C 2O 4) 3. Reaction of soluble lanthanum...
4 KB (251 words) - 21:05, 14 June 2025
however, amaranth leaves contain anti-nutritional factors, including oxalates, nitrates, saponins, and phenolic compounds. Cooking methods such as boiling...
17 KB (1,438 words) - 22:17, 3 February 2025
Nitrostarch (redirect from Starch nitrate)
Much like starch, it is made up of two components, nitrated amylose and nitrated amylopectin. Nitrated amylopectin generally has a greater solubility than...
4 KB (274 words) - 21:07, 23 May 2025
(2018). "Effects of Cooking Conditions on the Relationships Among Oxalate, Nitrate, and Lutein in Spinach". Food Science and Technology Research. 24 (3):...
9 KB (887 words) - 05:51, 6 July 2023
thorium nitrate solution, insoluble thorium oxalate precipitates. Other organic acids added to thorium nitrate solution produce precipitates of organic salts...
13 KB (1,237 words) - 21:48, 18 October 2024
It may be prepared by the metathesis reaction between lead(II) nitrate and sodium oxalate: Pb2+(aq) + C2O42− → PbC2O4 (s) A dihydrate may be formed with...
5 KB (414 words) - 15:58, 24 March 2025
fluoride Cobalt(III) chloride Cobalt(III) hydroxide Iron(III) nitrate Iron(III) oxalate W. Levason and C. A. McAuliffe (1974): "Higher oxidation state...
3 KB (321 words) - 17:07, 9 February 2025
space group. Uranyl oxalate trihydrate can be produced by the reaction of uranyl nitrate hexahydrate with oxalic acid. Uranyl oxalate has been used in actinometers...
3 KB (248 words) - 21:55, 26 May 2025
print. It has even been called kallitype, however that process uses ferric oxalate instead of ferric ammonium citrate. Concerns have been voiced about the...
2 KB (242 words) - 23:09, 25 December 2024
Ceric ammonium nitrate (CAN) is the inorganic compound with the formula (NH4)2[Ce(NO3)6]. This orange-red, water-soluble cerium salt is a specialised...
11 KB (894 words) - 21:15, 24 May 2025
temperature, both by increasing it (e.g. nitrates, chlorates) and decreasing it (e.g. carbonates, oxalates), indirectly influencing the flame brightness...
15 KB (581 words) - 22:40, 23 April 2025
Ba(OH)2 Barium iodide – BaI2 Barium manganate – BaMnO4 Barium nitrate – Ba(NO3)2 Barium oxalate – Ba(C2O4) Barium oxide – BaO Barium permanganate – Ba(MnO4)2...
122 KB (9,014 words) - 22:52, 20 June 2025
unstable. Uranyl oxalate has been used historically but is very toxic and cumbersome to analyze. Recent investigations into nitrate photolysis have used...
6 KB (707 words) - 13:52, 24 May 2025
may be eaten cooked or raw, and the taste differs considerably; the high oxalate content may be reduced by steaming. It is an annual plant (rarely biennial)...
19 KB (2,003 words) - 15:51, 24 May 2025
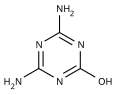
NH3 Ammeline is weakly acidic with pKa ~9. It can form nitrate, sulfate, chromate, and oxalate salts. Ammeline reacts with boiling dilute hydrochloric...
3 KB (114 words) - 14:53, 5 January 2024
Actinium(III) nitrate is an inorganic compound, actinium salt of nitric acid with the chemical formula Ac(NO3)3. The compound looks like white substance...
3 KB (180 words) - 23:39, 27 May 2025
non-spore-forming, oxalate-degrading anaerobic bacterium that was first isolated from human fecal samples. O. aliiformigenes consumes oxalate as its main carbon...
8 KB (837 words) - 21:48, 19 June 2025
Cadmium oxalate is an inorganic compound with the chemical formula CdC2O4. It can be produced by reacting potassium oxalate and cadmium nitrate. Its thermal...
3 KB (264 words) - 13:31, 24 May 2025
americium(III) oxalate crystals; once complete precipitation is achieved, additional oxalic acid is added to make a slurry. The slurry of americium oxalate and oxalic...
6 KB (591 words) - 05:56, 15 November 2024
IMA2018-032a) 8.0 [172] [no] [no] Natroxalate (oxalate: IMA1994-053) 10.AB.60 [173] [174] [175] (IUPAC: disodium oxalate) Natrozippeite (zippeite: IMA1971-004)...
39 KB (2,824 words) - 17:47, 9 February 2024
neodymium-praseodymium, samarium, gadolinium and yttrium in oxide and oxalate form (with more than 99% Purity). RED also produces strategic materials...
21 KB (2,097 words) - 13:13, 17 June 2025
Common counterions include chloride, bromide, sulfate, phosphate, nitrate, acetate, oxalate, citrate, and tartrate. Amine salts formed from the acid–base...
7 KB (875 words) - 19:07, 25 April 2025
acid-detergent fiber: 20.1–48.8% acid-detergent lignin: 3.1–10.4% nitrate: 0.1–6.2% water-soluble oxalate: 0.2–9.1% These figures are not the only important genetically...
36 KB (4,606 words) - 23:33, 7 May 2025