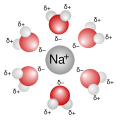
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group −OH), a nitrogen (as in an amine group...
3 KB (165 words) - 23:54, 21 December 2023
protic and aprotic. Protic solvents, such as water, solvate anions (negatively charged solutes) strongly via hydrogen bonding. Polar aprotic solvents...
47 KB (3,837 words) - 04:56, 28 June 2025
changing from a protic solvent to an aprotic solvent. This difference arises from acid/base reactions between protic solvents (not aprotic solvents) and strong...
17 KB (1,904 words) - 19:14, 18 October 2024
aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents, these...
4 KB (188 words) - 11:12, 25 June 2025
nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia...
8 KB (750 words) - 08:37, 2 May 2025
a racemic product. It is important to use a protic solvent, water and alcohols, since an aprotic solvent could attack the intermediate and cause unwanted...
13 KB (1,536 words) - 08:50, 10 May 2025
Molecular autoionization (section Protic solvents)
reaction remains unchanged. Such autoionization can be protic (H+ transfer), or non-protic. Protic solvents often undergo some autoionization (in this case autoprotolysis):...
4 KB (431 words) - 11:22, 7 March 2025
SN2 reaction (section Solvent)
water, and I− is a better nucleophile than Br− (in polar protic solvents). In a polar aprotic solvent, nucleophilicity increases up a column of the periodic...
21 KB (2,560 words) - 11:49, 26 June 2025
Solvation (redirect from Ion-solvent interaction)
accept H-bonds, donate H-bonds, or both. Solvents that can donate H-bonds are referred to as protic, while solvents that do not contain a polarized bond to...
19 KB (2,405 words) - 05:12, 24 June 2025
Acetic acid (category Solvents)
As a polar protic solvent, acetic acid is frequently used for recrystallization to purify organic compounds. Acetic acid is used as a solvent in the production...
63 KB (6,653 words) - 01:13, 24 June 2025
solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute in a solution. When the solvent is water it is called...
6 KB (642 words) - 02:27, 25 May 2025
SN1 reaction (section Solvent effects)
the reaction. The normal solvents of choice are both polar (to stabilize ionic intermediates in general) and protic solvents (to solvate the leaving group...
15 KB (1,978 words) - 11:47, 26 June 2025
Stevens Stevens (1900–2000). The usage of aprotic solvents gives predominantly Z-alkenes, while protic solvent gives a mixture of E- and Z-alkenes. As an alkene-generating...
14 KB (1,492 words) - 01:22, 26 June 2025
Acetonitrile (category Solvents)
it is used as a medium-polarity non-protic solvent that is miscible with water and a range of organic solvents, but not saturated hydrocarbons. It has...
21 KB (1,797 words) - 19:02, 4 February 2025
Aliquat 336 (section Solvent extraction of metals)
Properties of an Hydrophobic Ionic Liquid (Aliquat 336) in a Polar Protic Solvent (Formamide) at Different Temperatures". Journal of Dispersion Science...
6 KB (354 words) - 13:42, 23 April 2025
Thiodiglycol (redirect from Kromfax solvent)
2-chloroethanol with sodium sulfide. Thiodiglycol is a polar protic solvent. It is used as a solvent in a variety of applications ranging from dyeing textiles...
4 KB (313 words) - 13:13, 13 April 2025
February 1886 – 18 August 1965). Traditionally, an acidic catalyst in protic solvent was employed with heating; however, the reaction has been shown to work...
11 KB (1,187 words) - 07:01, 26 June 2025
number is related to the broader concept of solvation number, the number of solvent molecules bonded to a central atom. The hydration number varies with the...
10 KB (1,206 words) - 09:32, 4 December 2024
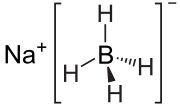
Sodium borohydride is soluble in protic solvents such as water and lower alcohols. It also reacts with these protic solvents to produce H2; however, these...
29 KB (2,764 words) - 19:20, 21 June 2025
transfer catalysis is very common. A wide range of solvents can be used, but protic solvents and apolar solvents tend to slow the reaction rate strongly, as...
11 KB (1,318 words) - 06:35, 26 June 2025
of oxygen interfere with the reaction path and reduce the yield). Protic solvents effect the Bouveault-Blanc ester reduction rather than condensation...
9 KB (926 words) - 09:30, 25 June 2025
temperature in matrix isolation experiments, or in certain nonpolar, non-protic solvents. Lithium superoxide is also a transient species during the reduction...
11 KB (1,208 words) - 20:28, 26 May 2025
Deep eutectic solvents or DESs are solutions of Lewis or Brønsted acids and bases which form a eutectic mixture. Deep eutectic solvents are highly tunable...
22 KB (2,553 words) - 05:59, 23 May 2025
nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions,...
10 KB (1,008 words) - 21:11, 11 December 2024
reduction through a series of electron transfer and proton transfer (from protic solvent) steps. Reducible functional groups include: α-Functionalized carbonyl...
17 KB (1,966 words) - 20:27, 29 August 2024
surfactant-based vesicle Oil dispersants – Mixture of emulsifiers and solvents used to treat oil spillsPages displaying short descriptions of redirect...
37 KB (3,984 words) - 20:42, 26 June 2025
Thus amides can participate in hydrogen bonding with water and other protic solvents; the oxygen atom can accept hydrogen bonds from water and the N–H hydrogen...
23 KB (2,336 words) - 00:53, 6 June 2025
Acid dissociation constant (section Mixed solvents)
10−7 M. A solvent will be more likely to promote ionization of a dissolved acidic molecule in the following circumstances: It is a protic solvent, capable...
103 KB (11,534 words) - 07:28, 2 June 2025
cyanoborohydride - a slightly stronger reductant, but amenable to protic solvents Sodium borohydride - a stronger, cheaper reductant Tetramethylammonium...
5 KB (412 words) - 01:11, 8 April 2025
C6H5C(O)CH3 + KH → C6H5C(O)CH2K + H2 Typical solvents for such reactions are ethers. Water and other protic solvents cannot serve as a medium for ionic hydrides...
21 KB (2,338 words) - 20:02, 19 June 2025