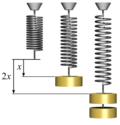
thermodynamics, a quasi-static process, also known as a quasi-equilibrium process (from Latin quasi, meaning ‘as if’), is a thermodynamic process that happens slowly...
4 KB (580 words) - 13:24, 19 March 2024
dissipation. To maintain equilibrium, reversible processes are extremely slow (quasistatic). The process must occur slowly enough that after some small...
13 KB (1,539 words) - 13:56, 6 April 2025
to think of the "processes" described by the paths as fictively "reversible". Reversible processes are always quasistatic processes, but the converse...
16 KB (2,191 words) - 14:54, 18 December 2024
temperature stays constant Polytropic process, which obeys the equation p v n = C {\displaystyle pv^{\,n}=C} Quasistatic process, which occurs infinitely slowly...
6 KB (686 words) - 04:06, 5 July 2024
Quasistatic can refer to: Quasistatic process Quasistatic equilibrium Quasistatic loading Quasistatic approximation This disambiguation page lists articles...
159 bytes (43 words) - 20:47, 29 December 2019
Thermodynamic cycle (redirect from Cyclic process)
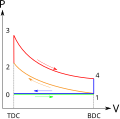
Cycles composed entirely of quasistatic processes can operate as power or heat pump cycles by controlling the process direction. On a pressure–volume...
19 KB (2,956 words) - 02:05, 11 April 2025
processes Cyclic process Isobaric process Isenthalpic process Isentropic process Isochoric process Isothermal process Polytropic process Quasistatic process...
44 KB (6,356 words) - 22:30, 22 February 2025
Adiabatic process Compressor Internal combustion engine Isentropic process Isobaric process Isochoric process Isothermal process Polytrope Quasistatic equilibrium...
7 KB (527 words) - 04:29, 10 March 2025
First law of thermodynamics (section Process of transfer of matter between an open system and its surroundings)
heat and work added, with no restrictions as to whether the process is reversible, quasistatic, or irreversible.[Warner, Am. J. Phys., 29, 124 (1961)] This...
95 KB (14,005 words) - 12:03, 7 May 2025
closer to the thermodynamical concept of a quasistatic process and has no direct relation with adiabatic processes in thermodynamics. In mechanics, an adiabatic...
20 KB (3,423 words) - 06:19, 17 April 2024
Adiabatic theorem (redirect from Adiabatic process (quantum mechanics))
for fast process. The classical and quantum mechanics definition is instead closer to the thermodynamical concept of a quasistatic process, which are...
46 KB (4,537 words) - 16:46, 14 May 2025
has been doubled. In the limit δV to zero, this becomes an ideal quasistatic process, albeit an irreversible one. Now, according to the fundamental thermodynamic...
17 KB (2,678 words) - 15:51, 28 December 2024
{\displaystyle \Delta F\leq W} , with equality holding only in the case of a quasistatic process, i.e. when one takes the system from A to B infinitely slowly (such...
11 KB (1,629 words) - 12:35, 7 November 2023
accepted that a process, compression or expansion, as desired, could be performed 'infinitely slowly'[,] or as is sometimes said, quasistatically." P. 130:...
15 KB (2,096 words) - 14:45, 15 January 2025
An isothermal process is a type of thermodynamic process in which the temperature T of a system remains constant: ΔT = 0. This typically occurs when a...
16 KB (2,276 words) - 09:25, 23 April 2025
an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which...
4 KB (527 words) - 16:48, 28 April 2025
An isenthalpic process or isoenthalpic process is a process that proceeds without any change in enthalpy, H; or specific enthalpy, h. If a steady-state...
3 KB (502 words) - 12:41, 13 May 2025
connection is that of quasistatic processes, namely idealized, "infinitely slow" processes. Time-dependent thermodynamic processes far away from equilibrium...
12 KB (1,643 words) - 03:10, 13 February 2025
In thermodynamics, an irreversible process is a process that cannot be undone. All complex natural processes are irreversible, although a phase transition...
20 KB (2,521 words) - 13:52, 6 April 2025
Adiabatic accessibility (category Thermodynamic processes)
The original definition of Carathéodory was limited to reversible, quasistatic process, described by a curve in the manifold of equilibrium states of the...
8 KB (1,224 words) - 20:17, 30 January 2025
path of a process through the equilibrium state space of a thermodynamic system is termed a process function, or, alternatively, a process quantity, or...
3 KB (390 words) - 12:18, 15 March 2025
can be approximated as adiabatic. Under these conditions and for quasistatic processes the first law of thermodynamics for a deformed body can be expressed...
56 KB (9,420 words) - 16:09, 7 May 2025
In thermodynamics, an isobaric process is a type of thermodynamic process in which the pressure of the system stays constant: ΔP = 0. The heat transferred...
12 KB (1,891 words) - 13:11, 17 November 2024
described by a Lorentzian profile and there may be an associated shift. Quasistatic pressure broadening: The presence of other particles shifts the energy...
20 KB (2,340 words) - 11:41, 24 May 2025
would flow at that voltage, say I = I ( V ) {\displaystyle I=I(V)} (the quasistatic approximation). Suppose further that the time to cross the device is...
3 KB (481 words) - 19:18, 22 April 2025
chemical composition and mass), d S = δ Q T (actually possible quasistatic irreversible process without composition change). {\displaystyle \mathrm {d} S={\frac...
107 KB (15,472 words) - 01:32, 4 May 2025
Heat capacity ratio (section Adiabatic process)
gives the important relation for an isentropic (quasistatic, reversible, adiabatic process) process of a simple compressible calorically-perfect ideal...
18 KB (2,324 words) - 16:04, 11 August 2024
Entropy (section Reversible process)
\mathrm {d} S={\frac {\delta Q_{\mathsf {rev}}}{T}}} The reversible process is quasistatic (i.e., it occurs without any dissipation, deviating only infinitesimally...
111 KB (14,228 words) - 21:07, 24 May 2025
Hampson–Linde cycle (redirect from Linde process)
The Hampson–Linde cycle is a process for the liquefaction of gases, especially for air separation. William Hampson and Carl von Linde independently filed...
6 KB (623 words) - 03:46, 17 August 2023
and isentropic processes (frictionless, adiabatic reversible). Left and right sides of the loop: a pair of parallel isochoric processes (constant volume)...
24 KB (4,251 words) - 12:47, 26 April 2025