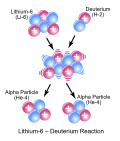The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration...
26 KB (3,727 words) - 13:22, 7 May 2025
expression for the reaction rate of a given reaction in terms of concentrations of chemical species and constant parameters (normally rate coefficients and...
44 KB (7,579 words) - 06:10, 25 May 2025
kinetics, a reaction rate constant or reaction rate coefficient ( k {\displaystyle k} ) is a proportionality constant which quantifies the rate and direction...
17 KB (2,387 words) - 12:44, 3 February 2025
adjustable rate, on-demand. Nuclear chain reactions in fissionable materials produce induced nuclear fission. Various nuclear fusion reactions of light...
20 KB (2,406 words) - 18:12, 7 February 2025
Enzyme kinetics (redirect from Enzyme reaction rate)
Enzyme kinetics is the study of the rates of enzyme-catalysed chemical reactions. In enzyme kinetics, the reaction rate is measured and the effects of varying...
72 KB (9,551 words) - 07:46, 27 March 2025
Chemical kinetics (redirect from Reaction kinetics)
also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different...
24 KB (3,326 words) - 20:37, 18 March 2025
and reaction conditions. Chemical reactions happen at a characteristic reaction rate at a given temperature and chemical concentration. Some reactions produce...
66 KB (8,057 words) - 19:52, 8 May 2025
Catalysis (redirect from Catalytic reaction)
increase in rate of a chemical reaction due to an added substance known as a catalyst (/ˈkætəlɪst/). Catalysts are not consumed by the reaction and remain...
46 KB (5,318 words) - 19:37, 27 May 2025
Transition state theory (redirect from Absolute reaction rate theory)
chemistry, transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium...
41 KB (5,712 words) - 03:35, 26 May 2025
involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement...
21 KB (2,555 words) - 13:23, 25 May 2025
applications. This reaction is accepted as a click chemistry reaction given the reactions' high yield, stereoselectivity, high rate, and thermodynamic...
25 KB (2,808 words) - 12:29, 23 May 2025
Nuclear fusion (redirect from Thermonuclear reaction)
Nuclear fusion is a reaction in which two or more atomic nuclei combine to form a larger nuclei, nuclei/neutron by-products. The difference in mass between...
113 KB (12,225 words) - 02:37, 28 May 2025
is an equation in electrochemical kinetics relating the rate of an electrochemical reaction to the overpotential. The Tafel equation was first deduced...
10 KB (1,161 words) - 09:00, 17 March 2025
Nucleophilic substitution (redirect from Nucleophilic substitution reaction)
affect the reaction rate of SN1 reactions. Instead of having two concentrations that affect the reaction rate, there is only one, substrate. The rate equation...
13 KB (1,536 words) - 08:50, 10 May 2025
the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation intermediate...
15 KB (1,970 words) - 03:54, 24 October 2024
chain reactions were proposed. It was understood that chemical chain reactions were responsible for exponentially increasing rates in reactions, such...
43 KB (6,129 words) - 18:55, 6 April 2025
overall rate of a reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting...
12 KB (1,736 words) - 20:02, 2 September 2024
Stellar nucleosynthesis (section Reaction rate)
and later by Edward Teller and Gamow himself to derive the rate at which nuclear reactions would occur at the high temperatures believed to exist in stellar...
38 KB (4,498 words) - 08:26, 24 May 2025
The erythrocyte sedimentation rate (ESR or sed rate) is the rate at which red blood cells in anticoagulated whole blood descend in a standardized tube...
25 KB (2,810 words) - 23:35, 23 May 2025
In chemistry, reaction progress kinetic analysis (RPKA) is a subset of a broad range of kinetic techniques utilized to determine the rate laws of chemical...
45 KB (4,772 words) - 20:47, 23 August 2023
Chemical equilibrium (redirect from Equilibrium reaction)
when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not...
44 KB (6,725 words) - 10:14, 16 May 2025
diffusion-limited) reactions are reactions in which the reaction rate is equal to the rate of transport of the reactants through the reaction medium (usually...
7 KB (1,364 words) - 05:18, 3 November 2024
its own rate law and molecularity. The sum of the elementary steps gives the net reaction. When determining the overall rate law for a reaction, the slowest...
14 KB (1,614 words) - 18:12, 24 May 2025
Thiele modulus (section Second order Reaction)
in Hill as hT. h T 2 = reaction rate diffusion rate {\displaystyle h_{T}^{2}={\dfrac {\mbox{reaction rate}}{\mbox{diffusion rate}}}} The derivation of...
4 KB (706 words) - 12:51, 25 November 2021
Half-life (redirect from Reaction half life)
for a zero order reaction depends on the initial concentration and the rate constant. In first order reactions, the rate of reaction will be proportional...
17 KB (2,175 words) - 05:07, 18 April 2025
Fenton's reagent (redirect from Fenton reaction)
reaction performance. Thus ongoing research has been done to optimize pH and amongst other parameters for greater reaction rates. The Fenton reaction...
16 KB (1,631 words) - 19:23, 25 May 2025
On-water reactions are a group of organic reactions that take place as an emulsion in water and have an unusual reaction rate acceleration compared with...
6 KB (784 words) - 06:12, 5 February 2025
Collision theory (section Rate equations)
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the reactant hit each...
19 KB (2,788 words) - 02:22, 31 May 2025
chemistry, the law of mass action is the proposition that the rate of a chemical reaction is directly proportional to the product of the activities or...
28 KB (3,544 words) - 14:45, 25 May 2025
Molecularity (redirect from Trimolecular reaction)
one product molecule. In either case, the rate of the reaction or step is described by the first order rate law d [ A ] d t = − k r [ A ] , {\displaystyle...
8 KB (1,137 words) - 15:22, 19 December 2024