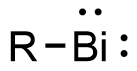Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new...
13 KB (1,441 words) - 22:05, 12 November 2024
of sulfones are typically reductive in nature. Olefination or replacement with hydrogen may be accomplished using reductive desulfonylation methods. The...
20 KB (2,256 words) - 14:31, 28 May 2025
Suzuki reaction (section Reductive elimination)
R1-Pdll-X (B). The final step is the reductive elimination step where the palladium(II) complex (E) eliminates the product (3) and regenerates the palladium(0)...
35 KB (3,984 words) - 17:56, 24 May 2025
Stille reaction (section Reductive elimination step)
catalyst (1), transmetalation of 3 with an organotin reagent (4), and reductive elimination of 5 to yield the coupled product (7) and the regenerated palladium...
47 KB (5,493 words) - 02:16, 16 May 2025
by reductive elimination. An unproductive side reaction can compete with reductive elimination wherein the amide undergoes beta-hydride elimination to...
31 KB (3,483 words) - 13:42, 28 March 2025
units in a process known as reductive elimination. (Confusingly, in organometallic terminology, the terms α-elimination and α-abstraction refer to processes...
14 KB (1,845 words) - 18:02, 18 June 2025
addition, transmetalation, and reductive elimination. Both systems also have to address issues of β-hydride elimination and difficult oxidative addition...
35 KB (4,130 words) - 14:11, 22 May 2025
In mathematics, Gaussian elimination, also known as row reduction, is an algorithm for solving systems of linear equations. It consists of a sequence of...
33 KB (4,369 words) - 22:29, 19 June 2025
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process...
10 KB (1,210 words) - 14:57, 11 January 2025
electrophilic partner. In such cases, the mechanism generally involves reductive elimination of R-R' from LnMR(R') (L = spectator ligand). This intermediate...
17 KB (1,499 words) - 03:56, 5 June 2025
ligands undergoing reductive elimination must be cis to each other or otherwise must rearrange to be cis before they can reductively eliminate. Finally, in...
29 KB (3,878 words) - 14:04, 29 June 2024
trans-metalation step is a bis-aryl palladium(II), which then undergoes reductive elimination to form the desired bis-aryl species as well as the starting Pd(0)...
25 KB (3,056 words) - 12:46, 6 February 2025
Reductive amination (also known as reductive alkylation) is a form of amination that converts a carbonyl group to an amine via an intermediate imine. The...
25 KB (2,483 words) - 15:19, 18 June 2025
these bonds once they are activated through oxidative addition and reductive elimination. This investigation provided detailed mechanisms, intermediates...
16 KB (1,672 words) - 15:44, 24 February 2025
complex C depends on the properties of the ligands. For the facile reductive elimination to occur, the substrate motifs need to be in close vicinity, i.e...
49 KB (5,463 words) - 19:06, 9 June 2025
elimination to form an alkene from the other carbon-rhodium bond leads to a rhodacyclohexanone intermediate with an exocyclic double bond. Reductive elimination...
19 KB (2,115 words) - 13:27, 12 June 2025
concerted manner. Allylic diazene rearrangement as the final step in the reductive 1,3-transposition of α,β-unsaturated tosylhydrazones to the reduced alkenes...
41 KB (4,214 words) - 14:06, 20 May 2025
are believed to proceed through a standard Pd (0/II) pathway with reductive elimination forging the key C-C bond. Deactivation of Pd(II) with excess cyanide...
7 KB (713 words) - 10:04, 28 May 2025
to give alkenes (olefins)(3) after alcohol functionalization and reductive elimination using sodium amalgam[1][2] or SmI2.[3] The reaction is named after...
12 KB (1,345 words) - 14:37, 26 May 2025
place, generating a copper(III) intermediate which then undergoes reductive elimination to generate the coupled product. Both of these mechanisms predict...
15 KB (1,732 words) - 05:45, 8 November 2023
Redox (redirect from Reduction (chemistry))
Nucleophilic abstraction Organic redox reaction Oxidative addition and reductive elimination Oxidative phosphorylation Partial oxidation Pro-oxidant Redox gradient...
35 KB (3,434 words) - 10:40, 3 May 2025
can undergo reductive elimination of the cross coupled product. Reduction of Ni(II) to Ni(0) by Mn closes the catalytic cycle. Reductive cross coupling...
6 KB (698 words) - 15:18, 22 June 2024
can undergo reductive elimination (3) to afford the desired isopropyl arene C. However, intermediate B can also undergo β-hydride elimination (4) to afford...
4 KB (254 words) - 00:36, 7 May 2025
bismuth(III)-ligand bonds facilitates ligand exchange and ultimately reductive elimination to release the product, reform the bismuthinidene, and create a...
40 KB (4,489 words) - 01:11, 27 May 2025
intermediate, which undergoes reductive elimination to forge the first C–C bond of the product. After β-carbon elimination of norbornene, the resultant...
11 KB (1,156 words) - 05:38, 28 May 2025
and water cases, the oxidative added product could also undergo reductive elimination, yielding O- or N-B bond formation. Carbene analogs Silylene Sasamori...
13 KB (1,329 words) - 15:12, 18 March 2025
to form the six-coordinate dicarbonyl complex, which undergoes reductive elimination to release acetyl iodide (CH3C(O)I). The catalytic cycle involves...
5 KB (491 words) - 15:25, 3 April 2025
stage a molecule of carbon monoxide is lost to 6. The final step is reductive elimination to form the cycloalkene. Organic Chemistry Portal Wikimedia Commons...
18 KB (1,816 words) - 08:49, 7 June 2025
kinetic studies implicate oxidative addition reaction followed by reductive elimination from Cu(III) intermediates (Ln = one or more spectator ligands):...
7 KB (811 words) - 05:09, 2 June 2025
copper(III)-aryl-alkoxide or copper(III)-aryl-amide intermediate undergoes Reductive elimination to give the aryl ether or aryl amine, respectively: Ar-Cu(III)-NHR-L2...
5 KB (623 words) - 12:55, 6 March 2025