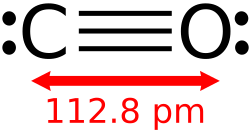Tin(II) oxide (stannous oxide) is a compound with the formula SnO. It is composed of tin and oxygen where tin has the oxidation state of +2. There are...
10 KB (912 words) - 13:29, 13 April 2025
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is...
15 KB (1,493 words) - 19:10, 24 May 2025
Tin oxide may refer to: Tin(II) oxide (stannous oxide), a black powder with the formula SnO Tin(IV) oxide (tin dioxide, stannic oxide), a white powder...
520 bytes (65 words) - 03:02, 4 August 2023
octaoate or 2-ethylhexanoate salt of tin. Produced by the reaction of tin(II) oxide and 2-ethylhexanoic acid, it is a clear colorless liquid at room temperature...
2 KB (157 words) - 21:08, 3 February 2025
oxidation by the air: 6 SnCl2 + O2 + 2 H2O → 2 SnCl4 + 4 Sn(OH)Cl Oxidation can be prevented by storing the solution over lumps of tin metal. Tin(II)...
17 KB (1,677 words) - 17:41, 24 May 2025
Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula PbO. It occurs in two polymorphs: litharge having a tetragonal...
14 KB (1,552 words) - 16:10, 26 May 2025
Sn(CH3COO)2. It was first discovered in 1822. To obtain tin(II) acetate, tin(II) oxide is dissolved in glacial acetic acid and refluxed to obtain yellow Sn(CH3COO)2·2CH3COOH...
3 KB (247 words) - 13:55, 28 May 2025
from tin industries, stannous oxide (SnO) and stannic oxide (SnO2), are specific to stannosis diagnoses. Hazardous occupations such as, tinning, tin-working...
10 KB (1,063 words) - 21:30, 17 December 2024
Monoxide (category Oxides)
neutral, germanium(II) oxide is distinctly acidic, and both tin(II) oxide and lead(II) oxide are amphoteric. Foundations of College Chemistry, 13th Edition...
1 KB (158 words) - 18:59, 18 November 2024
contains stannic oxide, SnO 2. Tin shows a chemical similarity to both of its neighbors in group 14, germanium and lead, and has two main oxidation states, +2...
83 KB (9,194 words) - 14:00, 26 May 2025
Neutrino Observatory Symphony Number One, an American chamber orchestra Tin(II) oxide (SnO) This disambiguation page lists articles associated with the title...
571 bytes (97 words) - 23:43, 4 January 2024
reacts with stannic oxide to give SnS at 450 °C: SnO2 + 2 KSCN → SnS + K2S + 2CO + N2 SnS also forms when aqueous solutions of tin(II) salts are treated...
10 KB (1,096 words) - 17:18, 25 May 2025
Carbon monoxide (redirect from Carbonic Oxide)
and one oxygen atom connected by a triple bond. It is the simplest carbon oxide. In coordination complexes, the carbon monoxide ligand is called carbonyl...
73 KB (7,597 words) - 19:47, 27 May 2025
Solder (redirect from Lead-tin solder)
structure: tin(IV) oxide on the surface, below it a layer of tin(II) oxide with finely dispersed lead, followed by a layer of tin(II) oxide with finely...
39 KB (4,925 words) - 17:45, 5 February 2025
process based on cerium(IV) oxide and cerium(III) oxide for hydrogen production. Cerium(III) oxide combined with tin(II) oxide (SnO) in ceramic form is used...
8 KB (733 words) - 02:26, 24 May 2025
Sn(OH)2 Tin(II) iodide – SnI2 Tin(II) oxide – SnO Tin(II) sulfate – SnSO4 Tin(II) sulfide – SnS Tin(IV) bromide – SnBr4 Tin(IV) chloride – SnCl4 Tin(IV) fluoride...
121 KB (8,924 words) - 03:42, 25 May 2025
for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. These compounds are known today as tin(II) oxide (SnO) and tin(IV) oxide (SnO2). In Dalton's...
9 KB (1,331 words) - 16:13, 11 September 2024
of basic solutions of tin(II). The compound consists of an octahedron of Sn centers, each face of which is capped by an oxide or a hydroxide. The structure...
3 KB (286 words) - 21:13, 22 March 2025
and forms crystalline hydrates. Effect of oxalic acid solution on tin(II) oxide : S n O + H 2 C 2 O 4 → S n C 2 O 4 ↓ + H 2 O {\displaystyle {\mathsf...
4 KB (373 words) - 01:58, 1 December 2023
reacting tin(II) oxide and nitric acid resulted in a formation of tin(II) nitrate hydroxide. This compound is sensitive to water, it hydrolyzes into tin(IV)...
6 KB (356 words) - 23:00, 17 February 2024
between the busbars must be flat. An electric current flows across the tin(II) oxide coating from one busbar to the other. The electrical resistance of the...
5 KB (631 words) - 21:53, 14 November 2024
germanium have been characterized. Tin(II) hydroxide Sn(OH)2 was prepared in anhydrous media. When tin(II) oxide is treated with alkali the pyramidal...
41 KB (4,863 words) - 18:23, 26 March 2025
for every 100 g of tin. 13.5 and 27 form a ratio of 1:2. These compounds are known today as tin(II) oxide (SnO) and tin(IV) oxide (SnO2). In Dalton's...
78 KB (10,108 words) - 00:25, 23 May 2025
Tin(IV) iodate is an inorganic compound with the chemical formula Sn(IO3)4. It was first obtained in 2020 through the hydrothermal reaction of tin(II)...
2 KB (170 words) - 20:03, 26 May 2025
Titanium oxide may refer to: Titanium dioxide (titanium(IV) oxide), TiO2 Titanium(II) oxide (titanium monoxide), TiO, a non-stoichiometric oxide Titanium(III)...
3 KB (333 words) - 22:13, 23 May 2025
Tin selenide, also known as stannous selenide, is an inorganic compound with the formula SnSe. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor...
18 KB (1,817 words) - 20:40, 7 March 2025
Silicon monoxide (redirect from Silicon(II) oxide)
chemical compound with the formula SiO where silicon is present in the oxidation state +2. In the vapour phase, it is a diatomic molecule. It has been...
14 KB (1,757 words) - 12:03, 7 January 2024
Tin foil, also spelled tinfoil, is a thin foil made of tin. Tin foil was superseded after World War II by cheaper and more durable aluminium foil, which...
2 KB (255 words) - 22:25, 28 March 2025
Zinc oxide is an inorganic compound with the formula ZnO. It is a white powder which is insoluble in water. ZnO is used as an additive in numerous materials...
85 KB (9,298 words) - 04:48, 2 June 2025
Tin telluride is a compound of tin and tellurium (SnTe); is a IV-VI narrow band gap semiconductor and has direct band gap of 0.18 eV. It is often alloyed...
6 KB (588 words) - 16:08, 29 May 2025