In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group R3Si−O−CR=CR2, composed of an enolate...
13 KB (1,369 words) - 22:56, 11 March 2025
enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are...
7 KB (807 words) - 11:17, 14 April 2025
used to install silyl groups onto hindered positions. Silyl triflate is more reactive and also converts ketones to silyl enol ethers. Silyl triflates are...
10 KB (1,269 words) - 23:02, 25 May 2025
electrophiles at oxygen. Silylation gives silyl enol ether. Acylation gives esters such as vinyl acetate. In general, enols are less stable than their keto equivalents...
14 KB (1,159 words) - 01:14, 13 March 2025
Ge, Sn, Pb). Such compounds are considered ethers as well. Examples of such ethers are silyl enol ethers R3Si−O−CR=CR2 (containing the Si−O−C linkage)...
19 KB (1,840 words) - 17:42, 18 July 2025
addition is an organic reaction and a type of aldol reaction between a silyl enol ether (R2C=CR−O−Si(CH3)3) and an aldehyde (R−CH=O) or formate (R−O−CH=O)...
9 KB (966 words) - 13:51, 25 June 2025
Like the aldol addition, the Michael reaction may proceed via an enol, silyl enol ether in the Mukaiyama–Michael addition, or more usually, enolate nucleophile...
27 KB (2,837 words) - 07:35, 18 July 2025
Silylation (redirect from Silyl)
identification. A common example of this is by trapping reactive enolates into silyl enol ethers, which represent reactive tautomers of many carbonyl compounds. The...
10 KB (1,072 words) - 16:26, 21 May 2025
Self-condensation (section Silyl enol ether formation)
will occur. One way to get around this is to turn the aldehyde into a silyl enol ether using trimethylsilyl chloride and a base, such as triethylamine, and...
4 KB (450 words) - 07:19, 26 June 2025
compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to...
13 KB (1,394 words) - 11:05, 8 August 2025
Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product...
28 KB (3,396 words) - 17:19, 26 September 2024
Aldol reaction (section On the enol)
uses other, similar functional groups as ersatz enols. In the Mukaiyama aldol reaction, silyl enol ethers add to carbonyls in the presence of a Lewis acid...
40 KB (4,255 words) - 19:40, 16 July 2025
1990). "Triethylborane induced perfluoroalkylation of silyl enol ethers or germyl enol ethers with perfluoroalkyl iodides". Tetrahedron Letters. 31 (44):...
46 KB (4,304 words) - 06:31, 25 July 2025
Locant (section Enols and enolates)
is in reaction with silyl chlorides, bromides, and iodides, where the oxygen acts as the nucleophile to produce silyl enol ether. IUPAC nomenclature Regioisomer...
7 KB (866 words) - 14:49, 28 February 2025
perform a Wittig reaction on acylsilane results in the formation of a silyl enol ether instead of the expected alkene, due to elimination by the carbanion...
7 KB (859 words) - 21:16, 15 July 2025
the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% yield. Lanthanide trifluoromethanesulfonates...
3 KB (181 words) - 06:27, 28 May 2025
agent, used to prepare derivatives of the type RCH2N(CH3)2. Enolates, silyl enol ethers, and even more acidic ketones undergo efficient dimethylaminomethylation...
5 KB (305 words) - 01:07, 8 March 2025
alkylation. They are also an important intermediate in the formation of silyl enol ether. Lithium enolate formation can be generalized as an acid–base reaction...
55 KB (5,955 words) - 23:00, 13 March 2025
epoxidation of alkenes (Prilezhaev reaction), conversion of silyl enol ethers to silyl α-hydroxy ketones (Rubottom oxidation), oxidation of sulfides...
6 KB (471 words) - 10:54, 23 July 2025
first step, a dirhodium catalyst effects diazo decomposition from silyl enol ether diazo compound to yield a donor/acceptor cyclopropene. The donor/acceptor...
6 KB (695 words) - 04:36, 10 October 2024
(CH3)3SiO3SCF3 is for the preparation of silyl enol ethers. One example involves the synthesis of the silyl enol ether of camphor: It was also used in Takahashi...
5 KB (450 words) - 08:59, 25 July 2025
preparation of α-fluoroketones from silyl enol ethers. Behaving like a pseudohalogen, it adds to ethylene to give the ether: CF3OF + CH2CH2 → CF3OCH2CH2F Cady...
3 KB (179 words) - 11:10, 10 June 2025
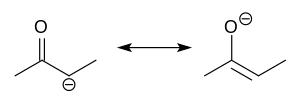
Enolate (category Enols)
can be trapped by acylation and silylation, which occur at oxygen. Silyl enol ethers are common reagents in organic synthesis as illustrated by the Mukaiyama...
21 KB (2,466 words) - 09:11, 19 May 2025
Reactions of allylsilanes are similar in many respects to reactions of silyl enol ethers—in fact, allylsilanes can be used as masked enolate equivalents. After...
12 KB (1,477 words) - 15:22, 7 February 2024
the Mukaiyama aldol addition reaction between benzaldehyde and the silyl enol ether of cyclohexanone with an 81% chemical yield. The corresponding reaction...
5 KB (538 words) - 16:54, 9 April 2025
sp-centered carbanions is limited to alkynylcuprates. Enolates and silyl enol ethers, the most widely used class of carbon nucleophiles in electrophilic...
18 KB (2,059 words) - 05:04, 23 May 2025
important; BINAP-AgF can be used to enantioselectively protonate silyl enol ethers. Subsequent studies revealed that related diphosphines with a narrower...
8 KB (583 words) - 15:42, 7 August 2025
titanium enolates. As a consequence, the double bond geometry in the silyl enol ether or allylsilane does not translate well into product stereochemistry...
45 KB (5,111 words) - 12:29, 27 June 2025
steps before elimination is carried out. (11) The combination of silyl enol ethers with palladium(II) acetate (Pd(OAc)2), the Saegusa oxidation, gives...
15 KB (1,586 words) - 18:56, 11 February 2025
(2006-06-01). "Catalytic Asymmetric Simmons−Smith Cyclopropanation of Silyl Enol Ethers. Efficient Synthesis of Optically Active Cyclopropanol Derivatives"...
29 KB (2,981 words) - 20:48, 22 May 2025