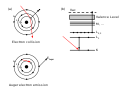
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic...
31 KB (4,013 words) - 11:21, 14 May 2025
Electron capture ionization is the ionization of a gas phase atom or molecule by attachment of an electron to create an ion of the form A − {\displaystyle...
9 KB (1,116 words) - 16:42, 22 September 2024
stripped of a single electron, and e− is the removed electron. Ionization energy is positive for neutral atoms, meaning that the ionization is an endothermic...
51 KB (5,833 words) - 19:31, 7 February 2025
solution for the ionization rate is possible. Tunnel ionization is ionization due to quantum tunneling. In classical ionization, an electron must have enough...
49 KB (6,848 words) - 12:03, 13 May 2025
Ion source (redirect from Atmospheric pressure ionization)
by the electron beam ion trap. Electron capture ionization (ECI) is the ionization of a gas phase atom or molecule by attachment of an electron to create...
58 KB (7,164 words) - 12:18, 20 February 2025
chemistry. Reagent gas molecules (often methane or ammonia) are ionized by electron ionization to form reagent ions, which subsequently react with analyte...
16 KB (2,132 words) - 10:15, 14 May 2025
Mass spectral interpretation (redirect from Even-electron rule)
is commonly used for the identification of organic compounds from electron ionization mass spectrometry. Organic chemists obtain mass spectra of chemical...
24 KB (2,827 words) - 18:35, 11 December 2023
complete seal). Electron ionization Ionization gauge Vacuum R. T. Bayard and D. Alpert, "Extension of the Low Pressure Range of the Ionization Gauge" Rev....
6 KB (785 words) - 21:52, 29 October 2024
atoms or molecules. Double ionization is usually less probable than single-electron ionization. Two types of double ionization are distinguished: sequential...
20 KB (1,970 words) - 10:02, 19 February 2025
Autoionization (redirect from Auto-ionization)
observed as variations in the ionization cross sections of atoms and molecules, by photoionization, electron ionization and other methods. As examples...
6 KB (720 words) - 14:01, 10 June 2024
manufacturers. The "hard ionization" process of electron ionization can be softened by the cooling of the molecules before their ionization, resulting in mass...
38 KB (4,826 words) - 08:13, 15 December 2024
Impact ionization is the process in a material by which one energetic charge carrier can lose energy by the creation of other charge carriers. For example...
4 KB (388 words) - 16:52, 6 April 2025
enough energy per individual photon or particle to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the...
60 KB (6,507 words) - 08:50, 15 May 2025
of electron capture ionization. The electron affinity is positive when energy is released on electron capture. In solid state physics, the electron affinity...
22 KB (1,468 words) - 11:02, 24 April 2025
molar ionization energy applies to the neutral atoms. The second, third, etc., molar ionization energy applies to the further removal of an electron from...
25 KB (229 words) - 07:16, 17 October 2024
In physics, tunnel ionization is a process in which electrons in an atom (or a molecule) tunnel through the potential barrier and escape from the atom...
24 KB (3,863 words) - 05:40, 27 August 2024
which each type utilises. The three basic types of gaseous ionization detectors are 1) ionization chambers, 2) proportional counters, and 3) Geiger–Müller...
8 KB (1,042 words) - 16:08, 23 July 2023
Japanese and it includes six types of spectra: laser Raman spectra, electron ionization mass spectra (EI-MS), Fourier-transform infrared (FT-IR) spectra...
8 KB (933 words) - 05:27, 12 March 2025
Mass spectrometry (redirect from Soft ionization)
isotope peaks). The most common example of hard ionization is electron ionization (EI). Soft ionization refers to the processes which impart little residual...
82 KB (10,342 words) - 10:57, 1 May 2025
Auger effect (redirect from Auger ionization)
the Auger electron corresponds to the difference between the energy of the initial electronic transition into the vacancy and the ionization energy for...
6 KB (714 words) - 03:47, 25 March 2025
The degree of ionization (also known as ionization yield in the literature) refers to the proportion of neutral particles, such as those in a gas or aqueous...
7 KB (764 words) - 17:34, 26 November 2023
desorption/ionization from a flat surface of target plate or, in other implementation, pulsed electron ionization or Resonance enhanced multiphoton ionization in...
4 KB (494 words) - 22:09, 24 January 2024
Ion (redirect from Free floating electrons)
electron in its lowest energy state from an atom or molecule of a gas with less net electric charge is called the ionization potential, or ionization...
31 KB (3,179 words) - 00:36, 26 April 2025
In physics, the Saha ionization equation is an expression that relates the ionization state of a gas in thermal equilibrium to the temperature and pressure...
16 KB (2,427 words) - 01:39, 4 May 2025
Thermal ionization mass spectrometry (TIMS), also known as surface ionization, is a highly sensitive isotope mass spectrometry characterization technique...
9 KB (1,056 words) - 00:21, 17 March 2025
process are ionized. Thermal ionization is used to make simple ion sources, for mass spectrometry and for generating ion beams. Thermal ionization has seen...
5 KB (652 words) - 21:11, 23 July 2024
break free from its associated atom's shell; this is ionization to form a positive ion. When an electron loses energy (thereby causing a photon to be emitted)...
24 KB (2,333 words) - 15:26, 27 November 2024
identities. The electron donating power of a donor molecule is measured by its ionization potential, which is the energy required to remove an electron from the...
5 KB (496 words) - 15:45, 20 January 2025
Penning ionization is a form of chemi-ionization, an ionization process involving reactions between neutral atoms or molecules. The Penning effect is...
8 KB (1,038 words) - 00:30, 30 May 2024
molecular, and optical physics, above-threshold ionization (ATI) is a multi-photon effect where an atom is ionized with more than the energetically required...
4 KB (475 words) - 02:51, 28 November 2023