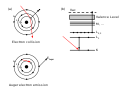
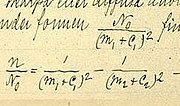In atomic physics and chemistry, an atomic electron transition (also called an atomic transition, quantum jump, or quantum leap) is an electron changing...
8 KB (868 words) - 11:15, 24 June 2025
Quantum jump (section Atomic electron transition)
used; as a rule scientists speak of transitions between quantum states or energy levels. Atomic electron transitions cause the emission or absorption of...
5 KB (522 words) - 20:12, 10 March 2024
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure)...
60 KB (6,208 words) - 21:30, 15 June 2025
Nuclear clock (category Atomic clocks)
an atomic clock being developed that will use the energy of a nuclear isomeric transition as its reference frequency, instead of the atomic electron transition...
52 KB (5,880 words) - 23:58, 20 March 2025
Atomic physics is the field of physics that studies atoms as an isolated system of electrons and an atomic nucleus. Atomic physics typically refers to...
14 KB (1,714 words) - 20:09, 4 June 2025
pyrotechnics and atomic emission spectroscopy. The color of the flames is understood through the principles of atomic electron transition and photoemission...
14 KB (990 words) - 01:27, 24 June 2025
associated with atomic electronic transitions and polyatomic gases have their own absorption band system. Atomic electron transition Resonance Raman spectroscopy...
5 KB (539 words) - 03:04, 24 August 2023
quantum mechanics, an atomic orbital (/ˈɔːrbɪtəl/ ) is a function describing the location and wave-like behavior of an electron in an atom. This function...
84 KB (10,931 words) - 22:10, 19 June 2025
Atom (redirect from Atomic chemical)
than electrons, it has a positive charge and is called a positive ion (or cation). The electrons of an atom are attracted to the protons in an atomic nucleus...
126 KB (12,975 words) - 23:44, 20 June 2025
of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. This process thereby changes a nuclear proton...
14 KB (1,274 words) - 02:07, 2 March 2025
Rydberg formula (category Atomic physics)
primarily presented as a generalization of the Balmer series for all atomic electron transitions of hydrogen. It was first empirically stated in 1888 by the Swedish...
13 KB (1,900 words) - 18:29, 23 June 2025
having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact...
113 KB (12,189 words) - 14:44, 24 June 2025
outermost isolated electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely...
33 KB (2,405 words) - 07:25, 24 May 2025
Aufbau principle (redirect from Principles in distribution of electrons)
the core electrons whose configuration in phosphorus is identical to that of neon. Electron behavior is elaborated by other principles of atomic physics...
28 KB (3,099 words) - 19:02, 17 June 2025
their unpaired d electrons, as are many of their compounds. All of the elements that are ferromagnetic near room temperature are transition metals (iron,...
40 KB (4,499 words) - 04:34, 21 June 2025
quantum Hall effect Transition radiation, contrasts to the Cherenkov radiation Atomic electron transition, the transition of an electron from one quantum...
8 KB (939 words) - 21:28, 20 May 2025
equal to the number of electrons. For an ordinary atom which contains protons, neutrons and electrons, the sum of the atomic number Z and the neutron...
21 KB (2,620 words) - 12:10, 5 June 2025
to the difference in energy levels between the electron energy states. Excited states in nuclear, atomic, and molecule systems have distinct energy values...
7 KB (821 words) - 21:31, 24 May 2025
Periodic table (redirect from Atomic table)
increasing atomic number. A new row (period) is started when a new electron shell has its first electron. Columns (groups) are determined by the electron configuration...
252 KB (27,180 words) - 17:20, 17 June 2025
spectrum. As the atomic number Z increases, so too does the number of potential Auger transitions. Fortunately, the strongest electron-electron interactions...
31 KB (4,117 words) - 02:55, 6 June 2025
leap (physics), also known as quantum jump, a transition between quantum states Atomic electron transition, a key example of the physics phenomenon Paradigm...
1 KB (207 words) - 00:03, 6 April 2025
Auger effect (redirect from Auger electrons)
a valence electron which is subsequently ejected from the atom. This second ejected electron is called an Auger electron. For heavier atomic nuclei, the...
6 KB (712 words) - 22:32, 5 June 2025
year (disambiguation) LEEP (disambiguation) Quantum leap, an Atomic electron transition https://leapinstitute.org This disambiguation page lists articles...
3 KB (345 words) - 23:58, 26 February 2025
core electrons of an atom form the atomic core. Core electrons are tightly bound to the nucleus. Therefore, unlike valence electrons, core electrons play...
10 KB (1,397 words) - 05:55, 19 June 2025
Rydberg formula. These observed spectral lines are due to the electron making transitions between two energy levels in an atom. The classification of the...
19 KB (1,910 words) - 14:26, 5 January 2025
Antihydrogen (section 1s–2s transition measurement)
and study H atoms. In 2016, the ALPHA experiment measured the atomic electron transition between the two lowest energy levels of antihydrogen, 1s–2s. The...
21 KB (2,217 words) - 17:47, 5 June 2025
electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...
14 KB (1,701 words) - 08:37, 25 May 2025
into these categories. Atomic physics is the subfield of AMO that studies atoms as an isolated system of electrons and an atomic nucleus, while molecular...
25 KB (2,972 words) - 14:55, 5 June 2025
Lyman-alpha (category Atomic physics)
(or, more generally, of any one-electron atom) in the Lyman series. It is emitted when the atomic electron transitions from an n = 2 orbital to the ground...
5 KB (496 words) - 22:05, 15 June 2025
electrons also facilitate all types of chemical reactions by being transferred or shared between atoms. The inner electron shells make up the atomic core...
155 KB (15,859 words) - 13:54, 24 June 2025