The bond valence method or mean method (or bond valence sum) (not to be mistaken for the valence bond theory in quantum chemistry) is a popular method in...
23 KB (3,337 words) - 20:25, 15 December 2024
chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods of quantum...
15 KB (1,887 words) - 16:20, 15 March 2025
valence bond (GVB) is a method in valence bond theory that uses flexible orbitals in the general way used by modern valence bond theory. The method was...
3 KB (325 words) - 16:21, 23 February 2023
Modern valence bond theory is the application of valence bond theory (VBT) with computer programs that are competitive in accuracy and economy, with programs...
20 KB (2,715 words) - 19:16, 15 April 2025
Molecular orbital theory (category Chemical bonding)
Molecular orbital theory and valence bond theory are the foundational theories of quantum chemistry. In the LCAO method, each molecule has a set of molecular...
28 KB (3,688 words) - 16:56, 11 May 2025
bonding: valence = number of electrons in valence shell of free atom − number of non-bonding electrons on atom in molecule, or equivalently: valence =...
40 KB (2,914 words) - 12:59, 11 January 2025
share "valence", such as is discussed in valence bond theory. In the molecule H 2, the hydrogen atoms share the two electrons via covalent bonding. Covalency...
29 KB (3,846 words) - 22:53, 13 April 2025
Heitler–London method forms the basis of what is now called valence bond theory. In 1929, the linear combination of atomic orbitals molecular orbital method (LCAO)...
40 KB (4,878 words) - 11:30, 7 May 2025
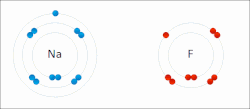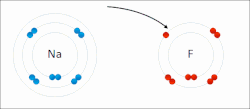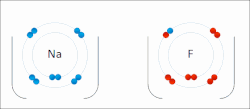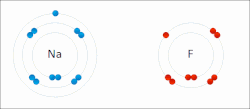
ionic bond results from the transfer of electrons from a metal to a non-metal to obtain a full valence shell for both atoms. Clean ionic bonding — in which...
18 KB (2,340 words) - 17:25, 5 February 2025
Orbital hybridisation (redirect from Sp² bond)
bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell...
33 KB (3,204 words) - 08:17, 15 March 2025
The covalent bond classification (CBC) method, also referred to as LXZ notation, is a way of describing covalent compounds such as organometallic complexes...
6 KB (734 words) - 09:09, 9 January 2024
In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom. An atom with a dangling bond is also referred to as an immobilized free...
25 KB (3,300 words) - 03:11, 27 December 2024
Lewis structure (category Chemical bonding)
achieved a valence shell electron configuration with a full octet of (8) electrons, hydrogen instead obeys the duplet rule, forming one bond for a complete...
16 KB (2,159 words) - 08:48, 30 April 2025
VSEPR theory (redirect from Valence shell electron pair repulsion)
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,059 words) - 16:52, 29 March 2025
Electronegativity (category Chemical bonding)
forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from...
35 KB (4,372 words) - 16:56, 11 May 2025
(the metals are off-center). The bond orders (valences), obtained from the bond lengths by the bond valence method, sum up to 2.01 at Fe and 3.99 at...
47 KB (11,970 words) - 15:23, 12 May 2025
Formal charge (redirect from Valence charge)
V is the number of valence electrons of the neutral atom in isolation (in its ground state); L is the number of non-bonding valence electrons assigned...
9 KB (1,050 words) - 02:14, 19 September 2024
stretched bonds. Bond order is also an index of bond strength and is also used extensively in valence bond theory. bond order = number of bonding electrons...
9 KB (1,285 words) - 19:04, 14 March 2025
Resonance (chemistry) (category Chemical bonding)
(or hybrid structure) in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one...
42 KB (5,100 words) - 06:18, 24 March 2025
Quantum chemistry (section Valence bond theory)
above, Heitler and London's method was extended by Slater and Pauling to become the valence-bond (VB) method. In this method, attention is primarily devoted...
19 KB (2,235 words) - 00:19, 5 May 2025
the valence electron clouds of the two rhenium atoms. From this data, we can conclude that this is a very strong bond. Experimentally, the Re-Re bond in...
9 KB (1,318 words) - 17:33, 21 April 2025
Molecular orbital (redirect from Gamma bond)
multiple atoms combine chemically into a molecule by forming a valence chemical bond, the electrons' locations are determined by the molecule as a whole...
35 KB (4,390 words) - 15:36, 2 January 2025
Valence bond (VB) computer programs for modern valence bond calculations:-[1] CRUNCH, by Gordon A. Gallup and his group. GAMESS (UK), includes calculation...
3 KB (395 words) - 12:21, 3 September 2024
coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two...
10 KB (1,313 words) - 13:10, 8 April 2025
Each valence bonding NBO σ must be paired with a corresponding valence antibonding NBO σ* (the acceptor) to complete the span of the valence space:...
5 KB (548 words) - 21:13, 16 October 2022
hybrid of the two methods—the popular B3LYP scheme is one such hybrid functional method. Another option is to use modern valence bond methods. For a list of...
31 KB (4,739 words) - 17:07, 14 April 2025
proposed. Current VB approaches are: Generalized valence bond (GVB) Modern valence bond theory (MVBT) A method that avoids making the variational overestimation...
21 KB (2,538 words) - 22:01, 26 January 2025
Hypervalent molecule (redirect from Hypervalent bonding)
for the bonding of hypervalent species, the energetic contribution of an expanded octet structure is also not null. In this modern valence bond theory...
39 KB (4,517 words) - 13:25, 25 April 2025
Electron counting (category Chemical bonding)
on the periodic table and determining the number of its valence electrons. One counts valence electrons for main group elements differently from transition...
14 KB (1,629 words) - 15:35, 19 January 2025
spin sizes other than 1/2. Just as one end of a valence bond is a spin 1/2, the ends of two valence bonds can be combined into a spin 1, three into a...
14 KB (1,815 words) - 16:34, 27 March 2025