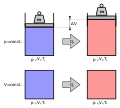
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R or R. It is the molar...
17 KB (1,839 words) - 00:58, 15 April 2025
The Boltzmann constant (kB or k) is the proportionality factor that relates the average relative thermal energy of particles in a gas with the thermodynamic...
26 KB (2,946 words) - 02:00, 12 March 2025
n} is the amount of substance; and R {\displaystyle R} is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved...
34 KB (4,623 words) - 08:36, 18 May 2025
volume of a gas to the amount of substance of gas present. Avogadro's law states that: The volume occupied by an ideal gas at a constant temperature is...
12 KB (1,770 words) - 12:56, 20 March 2025
Charles's law, the volume of gas is directly proportional to the temperature of that gas, when its pressure and mass are kept constant; that is, V ∝ T {\displaystyle...
3 KB (414 words) - 12:21, 14 May 2025
additive constant. The ideal quantum Boltzmann gas overcomes this limitation by taking the limit of the quantum Bose gas and quantum Fermi gas in the limit...
24 KB (3,821 words) - 02:41, 29 April 2025
Avogadro constant, NA, by n 0 = p 0 N A R T 0 , {\displaystyle n_{0}={\frac {p_{0}N_{\rm {A}}}{R\,T_{0}}},} where p0 is the pressure, R is the gas constant, and...
20 KB (2,297 words) - 17:01, 27 April 2025
temperature constant. He observed that when the pressure was increased in the gas, by adding more mercury to the column, the trapped gas' volume decreased...
52 KB (6,650 words) - 16:58, 5 May 2025
monatomic gas is 3 2 k B T N A = 3 2 R T {\displaystyle {\frac {3}{2}}k_{\text{B}}TN_{\text{A}}={\frac {3}{2}}RT} , where R is the gas constant. In an adiabatic...
4 KB (524 words) - 23:49, 28 February 2025
biochemical literature, as equilibrium constants. For an equilibrium mixture of gases, an equilibrium constant can be defined in terms of partial pressure...
42 KB (6,736 words) - 13:46, 8 March 2025
K_{\text{f}}={\frac {RMT_{\text{f}}^{2}}{1000\Delta H_{\text{fus}}}}} R is the ideal gas constant. M is the molar mass of the solvent. Tf is the freezing point of the...
2 KB (300 words) - 12:09, 19 March 2024
where h is the Planck constant and R the molar gas constant. As useful rules of thumb, a first-order reaction with a rate constant of 10−4 s−1 will have...
17 KB (2,387 words) - 12:44, 3 February 2025
Specific heat capacity (section Ideal gas)
substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated (specific heat capacity at constant pressure) than when...
55 KB (8,537 words) - 22:17, 8 April 2025
Heat capacity ratio (redirect from Poisson constant)
capacity (heat capacity per unit mass) of a gas. The suffixes P and V refer to constant-pressure and constant-volume conditions respectively. The heat capacity...
18 KB (2,324 words) - 16:04, 11 August 2024
such constants is also such a constant, such as the molar gas constant R {\displaystyle R} . List of mathematical constants Mathematical constant Physical...
32 KB (1,713 words) - 16:38, 26 September 2024
the ratio of specific heats at constant pressure and at constant volume (γ = Cp/Cv) and P is the pressure of the gas. For a closed system, one may write...
44 KB (6,356 words) - 22:30, 22 February 2025
Clausius–Clapeyron relation (redirect from Clausius–Clapeyron constant)
applies to vaporization of liquids where vapor follows ideal gas law using the ideal gas constant R {\displaystyle R} and liquid volume is neglected as being...
25 KB (3,651 words) - 04:27, 25 November 2024
Henry's law (redirect from Henry's Law constant)
excess dissolved gas is carried away by the blood and released into the lung gas. There are many ways to define the proportionality constant of Henry's law...
31 KB (4,896 words) - 13:18, 8 May 2025
The Loschmidt constant or Loschmidt's number (symbol: n0) is the number of particles (atoms or molecules) of an ideal gas per volume (the number density)...
6 KB (1,003 words) - 16:42, 4 December 2024
Stefan–Boltzmann law (redirect from Stefan–Boltzmann constant)
calculated from the measured value of the gas constant. The numerical value of the Stefan–Boltzmann constant is different in other systems of units, as...
38 KB (5,475 words) - 12:06, 7 May 2025
090 ft or 6.8 mi). From 11 km up to 20 km (65,620 ft or 12.4 mi), the constant temperature is −56.5 °C (−69.7 °F), which is the lowest assumed temperature...
30 KB (3,650 words) - 13:19, 18 April 2025
{\displaystyle T} , absolute temperature (K) R {\displaystyle R} is the gas constant, 8.31446261815324 in J⋅K−1⋅mol−1 M {\displaystyle M} is the molar mass...
18 KB (2,864 words) - 12:35, 30 April 2025
Avogadro's law (category Gas laws)
the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant. The law is named after Amedeo Avogadro...
10 KB (1,395 words) - 18:07, 19 May 2025
Fick's laws of diffusion (redirect from Diffusion constant)
the ith species, c is the concentration (mol/m3), R is the universal gas constant (J/K/mol), T is the absolute temperature (K), μ is the chemical potential...
58 KB (8,249 words) - 04:55, 19 April 2025
simpler kinetic theory of gases, we expect the heat capacity of a monatomic ideal gas to be constant, since for such a gas only kinetic energy contributes...
8 KB (1,232 words) - 18:28, 2 December 2024
gas equals cv T, where T is absolute temperature and the specific heat at constant volume is cv = (f)(R/2). R = 8.314 J/(K mol) is the universal gas constant...
17 KB (2,360 words) - 15:02, 12 May 2025
between the molar heat capacity at constant pressure and the molar heat capacity at constant volume for an ideal gas. Mayer's relation states that C P...
3 KB (437 words) - 20:10, 29 March 2025
In chemistry, an acid dissociation constant (also known as acidity constant, or acid-ionization constant; denoted K a {\displaystyle K_{a}} ) is a...
103 KB (11,531 words) - 14:03, 17 May 2025
Trouton's rule (redirect from Trouton's constant)
after Frederick Thomas Trouton. It is expressed as a function of the gas constant R: Δ S ¯ vap ≈ 10.5 R . {\displaystyle \Delta {\bar {S}}_{\text{vap}}\approx...
6 KB (649 words) - 02:56, 25 February 2025
{\displaystyle n} is the number of moles of the gas, R {\displaystyle R} is the universal gas constant, γ {\displaystyle \gamma } is the heat capacity...
20 KB (2,906 words) - 18:44, 29 April 2025