The Hammett acidity function (H0) is a measure of acidity that is used for very concentrated solutions of strong acids, including superacids. It was proposed...
8 KB (949 words) - 17:08, 7 March 2025
notably the Hammett acidity function, H0, for superacid media and its modified version H− for superbasic media. The term acidity function is also used...
5 KB (570 words) - 09:49, 28 May 2025
definition) is an acid with an acidity greater than that of 100% pure sulfuric acid (H2SO4), which has a Hammett acidity function (H0) of −12. According to...
13 KB (1,515 words) - 08:12, 15 April 2025
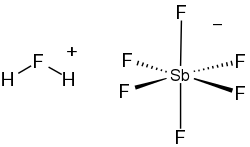
Fluoroantimonic acid (section Acidity)
pure sulfuric acid, by many orders of magnitude, according to its Hammett acidity function. It even protonates some hydrocarbons to afford pentacoordinate...
16 KB (1,578 words) - 14:55, 29 May 2025
Carborane acid (section Acidity)
times stronger than 100% pure sulfuric acid in terms of their Hammett acidity function values (H0 ≤ −18) and possess computed pKa values well below −20...
23 KB (2,548 words) - 05:34, 23 May 2025
Acid dissociation constant (redirect from Acidity constant)
convenient acidity function. Other acidity functions have been proposed for non-aqueous media, the most notable being the Hammett acidity function, H0, for...
103 KB (11,534 words) - 07:28, 2 June 2025
of a scheme for comparing their acidities based on what is now known as the Hammett acidity function. The Curtin–Hammett principle bears his name. The awards...
4 KB (227 words) - 12:33, 15 May 2024
solute (most commonly a weak aniline base) is measured by its Hammett acidity function, the H 0 {\displaystyle H_{0}} value. Although these two concepts...
19 KB (2,474 words) - 21:52, 17 April 2025
the Hammett acidity function. For instance, sulfuric acid, H2SO4, has a Hammett acidity function, H0, of −12, perchloric acid, HClO4, has a Hammett acidity...
16 KB (1,747 words) - 18:24, 25 March 2025
Hydrofluoric acid (section Acidity)
formation of other polymeric species, H n−1F− n, is highly likely. The Hammett acidity function, H0, for 100% HF was first reported as −10.2, while later compilations...
22 KB (2,171 words) - 13:49, 27 April 2025
H3BO3 Perchloric acid HClO4 Hydrogen cyanide HCN Boyd, Claude E. (2020). "Acidity". Water Quality: 215–231. doi:10.1007/978-3-030-23335-8_11. ISBN 978-3-030-23334-1...
2 KB (248 words) - 02:34, 22 April 2025
carbonic acid) when dissolved. Generally non-metallic oxides are acidic. The acidity of an oxide can be reasonably assumed by its accompanying constituents...
5 KB (592 words) - 21:19, 19 May 2025
PH indicator (redirect from Acidity or alkalinity)
halochromic chemical compound added in small amounts to a solution so the pH (acidity or basicity) of the solution can be determined visually or spectroscopically...
19 KB (1,671 words) - 05:48, 19 April 2025
in rail transport modelling Higgs boson, in physics, symbol H0 Hammett acidity function, in chemistry, H0 Hubble constant, in cosmology, H0 Null hypothesis...
647 bytes (102 words) - 01:25, 25 October 2023
hydronium/lyonium ion. pH is an example of an acidity function, but others can be defined. For example, the Hammett acidity function, H0, has been developed in connection...
53 KB (6,540 words) - 15:50, 17 June 2025
strength Acidity function Amphoterism Base Buffer solutions Dissociation constant Donor number Equilibrium chemistry Extraction Hammett acidity function pH...
12 KB (1,185 words) - 19:40, 11 June 2025
Proton affinity (redirect from Gas-phase acidity)
affinities illustrate the role of hydration in aqueous-phase Brønsted acidity. Hydrofluoric acid is a weak acid in aqueous solution (pKa = 3.15) but...
15 KB (1,028 words) - 15:15, 24 April 2024
Dashiell Hammett Hammett Prize Hammett, Georgia, a community in the United States Hammett acidity function Hammett equation, based on the Hammett substituent...
1 KB (197 words) - 04:08, 5 October 2020
Lewis acids and bases (redirect from Lewis acidity)
emphasize the kinetic aspect of reactivity, while the Lewis basicity and Lewis acidity emphasize the thermodynamic aspect of Lewis adduct formation. In many cases...
22 KB (2,774 words) - 08:02, 25 May 2025
strength Acidity function Amphoterism Base Buffer solutions Dissociation constant Donor number Equilibrium chemistry Extraction Hammett acidity function pH...
3 KB (335 words) - 11:18, 3 July 2023
properties. The most common organic acids are the carboxylic acids, whose acidity is associated with their carboxyl group –COOH. Sulfonic acids, containing...
10 KB (1,203 words) - 20:01, 24 May 2025
strength Acidity function Amphoterism Base Buffer solutions Dissociation constant Donor number Equilibrium chemistry Extraction Hammett acidity function pH...
9 KB (1,368 words) - 08:10, 2 June 2025
chemists seek the proton-removing ability of a base without any other functions. Typical non-nucleophilic bases are bulky, such that protons can attach...
3 KB (354 words) - 02:44, 22 March 2024
as a weak base, reacting with Lewis acids to give superacids. A Hammett acidity function (H0) of −21 is obtained with antimony pentafluoride (SbF5), forming...
15 KB (1,382 words) - 16:14, 31 May 2025
strength Acidity function Amphoterism Base Buffer solutions Dissociation constant Donor number Equilibrium chemistry Extraction Hammett acidity function pH...
5 KB (573 words) - 16:25, 10 February 2025
solution. In biological systems this is an essential condition for enzymes to function correctly. For example, in human blood a mixture of carbonic acid (H 2CO...
22 KB (2,336 words) - 05:58, 25 May 2025
− {\displaystyle {\ce {NH3 + H2O -> NH4+ + OH-}}} From this, a pH, or acidity, can be calculated for aqueous solutions of bases. A base is also defined...
26 KB (3,045 words) - 01:37, 24 May 2025
Non-Aqueous Solvents. Pergamon Press. Reich, Hans J. "Bordwell pKa Table (Acidity in DMSO)". Department of Chemistry, University of Wisconsin, U.S. Archived...
16 KB (1,949 words) - 16:30, 29 May 2025
method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (Et3PO, TEPO) is used as...
7 KB (972 words) - 06:42, 20 May 2025