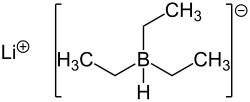
Lithium aluminium hydride, commonly abbreviated to LAH, is an inorganic compound with the chemical formula Li[AlH4] or LiAlH4. It is a white solid, discovered...
35 KB (3,062 words) - 17:08, 29 May 2025
Lithium hydride is an inorganic compound with the formula LiH. This alkali metal hydride is a colorless solid, although commercial samples are grey. Characteristic...
19 KB (1,922 words) - 10:36, 4 June 2025
generated in THF from lithium aluminium hydride. The reaction with lithium hydride in ether produces lithium aluminium hydride: AlH3 + LiH → Li[AlH4]...
29 KB (2,940 words) - 16:52, 28 May 2025
metal hydrides react with metal halides. Lithium aluminium hydride (often abbreviated as LAH) arises from reactions of lithium hydride with aluminium chloride...
21 KB (2,337 words) - 19:47, 20 April 2025
reagent, SMEAH is comparable with lithium aluminium hydride (LAH, LiAlH4). It is a safer alternative to LAH and related hydrides. SMEAH exhibits similar reducing...
5 KB (381 words) - 21:02, 6 August 2024
Diborane (redirect from Boron hydride)
sodium hydride (NaH), lithium hydride (LiH) or lithium aluminium hydride (LiAlH4): 8 BF3 + 6 LiH → B2H6 + 6 LiBF4 Lithium hydride used for this purpose must...
27 KB (2,667 words) - 05:23, 25 May 2025
soluble in ethers, whilst remaining safer to handle than lithium aluminium hydride. Lithium borohydride may be prepared by the metathesis reaction, which...
10 KB (833 words) - 02:07, 31 October 2023
stronger reducing agent than lithium borohydride and lithium aluminium hydride. LiBHEt3 is prepared by the reaction of lithium hydride (LiH) and triethylborane...
8 KB (588 words) - 21:20, 23 May 2025
refer to the reduction of a ketone by lithium aluminium hydride, but not to the oxidation of lithium aluminium hydride by a ketone. Many oxidations involve...
6 KB (672 words) - 19:09, 3 March 2024
Carbonyl reduction (redirect from Hydride reduction)
(nitro group, nitrile, ester). In their handling properties, lithium aluminium hydride and sodium borohydride (and their derivatives) strongly differ...
24 KB (2,729 words) - 15:28, 19 January 2025
Metal hydrides (sodium hydride, lithium aluminium hydride, uranium trihydride) Partially or fully alkylated derivatives of metal and nonmetal hydrides (diethylaluminium...
9 KB (796 words) - 20:03, 1 June 2025
suspension in diethyl ether, it reacts with lithium chloride to give the popular reagent lithium aluminium hydride: LiCl + NaAlH4 → LiAlH4 + NaCl The compound...
5 KB (456 words) - 10:28, 18 September 2024
Mg(anthracene) + H2 → MgH2 the reaction of diethylmagnesium with lithium aluminium hydride product of complexed MgH2 e.g. MgH2.THF by the reaction of phenylsilane...
12 KB (1,102 words) - 04:09, 21 April 2025
include lithium aluminium hydride (LiAlH4), lithium triethylborohydride, n-butyllithium and tert-butyllithium. Metallic lithium and its complex hydrides, such...
146 KB (14,240 words) - 04:46, 26 May 2025
synthesized in 1951 by treating dimethylberyllium, Be(CH3)2, with lithium aluminium hydride, LiAlH4. Purer BeH2 forms from the pyrolysis of di-tert-butylberyllium...
9 KB (781 words) - 23:33, 18 December 2024
agents for the non-catalytic conversion to amines include lithium aluminium hydride, lithium borohydride, diborane, or elemental sodium in alcohol solvents...
7 KB (738 words) - 21:47, 29 July 2022
important aluminium hydride is lithium aluminium hydride (LiAlH4), which is used as a reducing agent in organic chemistry. It can be produced from lithium hydride...
141 KB (15,298 words) - 14:15, 4 June 2025
aluminium hydride. Lithium tetrahydridogallate was first reported by Finholt, Bond and Schlesinger. It is prepared by the reaction of lithium hydride and...
6 KB (652 words) - 13:10, 28 May 2025
synthesis include the reaction of polonium tetrachloride (PoCl4) with lithium aluminium hydride (LiAlH4), which only produces elemental polonium, and the reaction...
6 KB (540 words) - 16:07, 27 January 2024
azetidinones (β-lactams) with lithium aluminium hydride. Even more effective is a mixture of lithium aluminium hydride and aluminium trichloride, a source of...
5 KB (316 words) - 14:33, 28 April 2025
a medical qualification awarded in Dublin, Ireland until 1968 Lithium aluminium hydride, an inorganic compound used as a reducing agent 1st SS Panzer...
890 bytes (143 words) - 12:51, 14 April 2023
Sodium borohydride (redirect from Sodium boro hydride)
tri-sec-butylborohydride), a more strongly reducing derivative. Lithium aluminium hydride, a more strongly reducing reagent, capable of reducing esters...
29 KB (2,764 words) - 15:53, 26 May 2025
hydrogenation are practiced in refineries. They can be effected by using lithium aluminium hydride, Clemmenson reduction and other specialized routes. Coal is a...
63 KB (7,004 words) - 08:31, 4 June 2025
nitro compounds with metal hydrides gives good yields of azo compounds. For example, one could use: Lithium aluminium hydride Zinc metal with sodium hydroxide...
14 KB (1,485 words) - 04:45, 25 May 2025
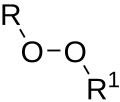
similar fashion. Organoperoxides can be reduced to alcohols with lithium aluminium hydride, as described in this idealized equation: 4 ROOH + LiAlH4 → LiAlO2...
23 KB (2,460 words) - 13:47, 27 May 2025
Zinc(II) hydride was first synthesized in 1947 by Hermann Schlesinger, via a reaction between dimethylzinc Zn(CH3)2 and lithium aluminium hydride Li[AlH4];...
8 KB (910 words) - 14:00, 6 May 2025
amines using lithium aluminium hydride. Azoalkanes may be reduced to primary amines by Staudinger reduction or lithium aluminium hydride. Amines may also...
20 KB (2,474 words) - 17:25, 30 May 2025
These parts (functional groups) must be protected. For example, lithium aluminium hydride is a highly reactive reagent that usefully reduces esters to alcohols...
57 KB (6,755 words) - 19:03, 31 March 2025
Hydrogen storage (section Metal hydrides)
hydrogen storage densities. Leading candidates are lithium hydride, sodium borohydride, lithium aluminium hydride and ammonia borane. A French company McPhy Energy...
135 KB (14,025 words) - 04:57, 28 May 2025
laboratory scale reactions it was made obsolete by the introduction of lithium aluminium hydride. Sodium metal is a one-electron reducing agent. Four equivalents...
8 KB (736 words) - 02:03, 23 April 2025