Lithium superoxide is an unstable inorganic salt with formula LiO2. A radical compound, it can be produced at low temperature in matrix isolation experiments...
11 KB (1,208 words) - 20:28, 26 May 2025
Curtiss, Larry A.; Amine, Khalil (January 2016). "A lithium–oxygen battery based on lithium superoxide". Nature. 529 (7586): 377–382. doi:10.1038/nature16484...
52 KB (5,758 words) - 18:45, 15 June 2025
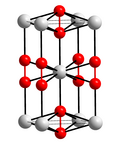
Potassium superoxide is an inorganic compound with the formula KO2. It is a yellow paramagnetic solid that decomposes in moist air. It is a rare example...
8 KB (634 words) - 01:27, 13 February 2025
Alkali metal (redirect from Lithium family)
potassium superoxide, while rubidium and caesium form the superoxide exclusively. Their reactivity increases going down the group: while lithium, sodium...
225 KB (24,164 words) - 20:18, 19 June 2025
Sodium superoxide is the inorganic compound with the formula NaO2. This yellow-orange solid is a salt of the superoxide anion. It is an intermediate in...
4 KB (283 words) - 16:02, 25 May 2025
(Cs4O6) is a black solid. Lithium superoxide (LiO2) has only been isolated in matrix isolation at 15 K. Sodium superoxide (NaO2) is a yellow-orange solid...
5 KB (457 words) - 06:12, 28 May 2025
the principal lithium mineral spodumene (LiAlSi2O6) is 8.03%. Lithium oxide forms along with small amounts of lithium peroxide when lithium metal is burned...
7 KB (483 words) - 03:49, 30 May 2025
Caesium superoxide is a chemical compound with the chemical formula CsO2. It consists of caesium cations Cs+ and superoxide anions O−2. It is an orange...
3 KB (195 words) - 11:21, 29 December 2023
Rubidium superoxide or rubidium hyperoxide is a chemical compound with the chemical formula RbO2. In terms of oxidation states, the negatively charged...
6 KB (489 words) - 16:56, 29 December 2023
Metal–air electrochemical cell (section Lithium)
associated with superoxide in lithium–air batteries. Sodium, with an energy density of 1605 Wh/kg, does not boast as high an energy density as lithium. However...
21 KB (2,244 words) - 19:57, 19 June 2025
oxygen via a chemical reaction. The oxygen source is usually an inorganic superoxide, chlorate, or perchlorate. Ozonides are a promising group of oxygen sources...
10 KB (1,144 words) - 23:55, 11 May 2025
"beyond-lithium-ion" batteries, such as lithium-sulfur and lithium–air batteries. He helped create a Li-O2 battery that runs on lithium superoxide. Curtiss and researchers...
15 KB (1,577 words) - 19:45, 22 May 2025
1310–65–2 LiO2 lithium superoxide 12136–56–0 LiPF6 lithium hexafluorophosphate 21324–40–3 LiPH2O4 lithium dihydrogenphosphate 13453–80–0 LiReO4 lithium perrhenate...
139 KB (120 words) - 17:15, 19 May 2025
– FeLiO4P Lithium nitrate – LiNO3 Lithium sulfide – Li2S Lithium sulfite – Li2SO3 Lithium sulfate – Li2SO4 Lithium superoxide – LiO2 Lithium hexafluorophosphate...
122 KB (9,014 words) - 22:52, 20 June 2025
built in 1873 in Berlin. Ozonide, O−3 Superoxide, O−2 Hydrogen peroxide Vol'nov, I. I. Peroxides, superoxides and ozonides of alkali and alkaline earth...
11 KB (1,287 words) - 16:56, 11 April 2025
Bijandra; Sun, Yang-Kook; Curtis, Larry (2007-01-11). "A lithium–oxygen battery based on lithium superoxide". Nature. 529 (1): 377–382. doi:10.1038/nature16484...
17 KB (1,297 words) - 23:37, 21 June 2025
sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical...
6 KB (716 words) - 00:03, 21 March 2025
studied: potassium oxide (K2O), potassium peroxide (K2O2), potassium superoxide (KO2) and potassium ozonide (KO3). The binary potassium-oxygen compounds...
97 KB (11,228 words) - 22:59, 22 May 2025
Electron transfer from a neutral lithium (Li) atom on the left to a neutral fluorine (F) atom on the right would give a Li+ and F− ions....
31 KB (3,198 words) - 16:00, 28 May 2025
poisonous soluble compounds which interfere with enzyme systems, such as superoxide dismutase, catalase, or glutathione peroxidase. Only soluble metal-containing...
21 KB (2,214 words) - 14:22, 23 May 2025
thus particularly useful in scuba gear, submarines, etc. Lithium peroxide and potassium superoxide have similar uses. Sodium peroxide was once used on a...
8 KB (599 words) - 23:38, 20 June 2025
reactive oxygen-derived compounds, including superoxide (which is produced by NADPH oxidase). Superoxide decays to oxygen and hydrogen peroxide, which...
18 KB (1,708 words) - 23:37, 24 May 2025
form superoxides. Li is the only group 1 element which forms a stable nitride, Li3N. Mg, as well as other group 2 elements, also form nitrides. Lithium carbonate...
5 KB (642 words) - 19:55, 16 August 2024
generated by radical-generating enzymes. In living organisms, the radicals superoxide and nitric oxide and their reaction products regulate many processes,...
41 KB (4,607 words) - 22:34, 15 June 2025
carbonyl compounds: aldehydes, ketones, peroxides. Ozonolysis Ozone cracking Superoxide, O− 2 Oxide, O2− Dioxygenyl, O+ 2 Cotton, F. A.; Wilkinson, G. (1988)...
7 KB (597 words) - 15:09, 24 May 2025
with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2). Potassium peroxide reacts with water to form potassium hydroxide...
3 KB (153 words) - 07:15, 1 March 2025
a type of battery and analogue to lithium-ion batteries, using potassium ions for charge transfer instead of lithium ions. It was invented by the Iranian/American...
19 KB (2,229 words) - 13:45, 24 May 2025
Rebreather (redirect from Superoxide scrubber)
been a few rebreather designs (e.g. the Oxylite) which use potassium superoxide, which gives off oxygen as it absorbs carbon dioxide, as the carbon dioxide...
85 KB (8,674 words) - 17:53, 24 May 2025
monoxide (Rb2O), Rb6O, and Rb9O2; rubidium in excess oxygen gives the superoxide RbO2. Rubidium forms salts with halogens, producing rubidium fluoride...
40 KB (4,531 words) - 06:36, 24 June 2025
Biometal (biology) (section Lithium)
being the production of hydroxyl radicals. Ferric ions can be reduced via superoxide and the product can be reoxidized via peroxide to form hydroxyl radicals...
23 KB (2,742 words) - 05:27, 2 June 2025