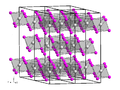
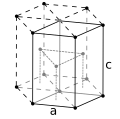
Lutetium(III) oxide, a white solid, is a cubic compound of lutetium sometimes used in the preparation of specialty glasses. It is also called lutecia...
5 KB (361 words) - 13:42, 15 July 2023
This page provides supplementary chemical data on Lutetium(III) oxide The handling of this chemical may incur notable safety precautions. It is highly...
5 KB (79 words) - 11:53, 12 April 2023
Yttrium oxide, also known as yttria, is Y2O3. It is an air-stable, white solid substance. The thermal conductivity of yttrium oxide is 27 W/(m·K). Yttrium...
9 KB (892 words) - 18:33, 5 April 2024
Lutetium compounds are compounds formed by the lanthanide metal lutetium (Lu). In these compounds, lutetium generally exhibits the +3 oxidation state...
16 KB (1,801 words) - 11:58, 24 May 2024
Holmium(III) oxide, or holmium oxide is a chemical compound of the rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III)...
14 KB (1,188 words) - 00:27, 18 April 2024
Lutetium(III) chloride or lutetium trichloride is the chemical compound composed of lutetium and chlorine with the formula LuCl3. It forms hygroscopic...
5 KB (216 words) - 11:16, 29 December 2023
Ytterbium(III) oxide is the chemical compound with the formula Yb2O3. It is one of the more commonly encountered compounds of ytterbium. It occurs naturally...
6 KB (442 words) - 15:27, 5 March 2024
3 H2O Lutetium(III) acetate also can be obtained by reacting lutetium oxide with gaseous acetic acid or 50% acetic acid solution. Lutetium(III) acetate...
5 KB (305 words) - 15:27, 28 May 2024
Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3. Lutetium(III) fluoride can be produced by reacting lutetium oxide with hydrogen...
4 KB (319 words) - 17:57, 18 August 2022
minimal lattice mismatch of 0.6%. The growth was done using powdered lutetium(III) oxide and crushed sapphire feedstock with 2M potassium bicarbonate mineralizer...
6 KB (727 words) - 12:50, 9 April 2024
lutetium(III) bromide may produce hydrogen bromide and metal oxide fumes. Lutetium(III) bromide reacts to strong oxidizing agents. An experiment by T...
5 KB (212 words) - 17:57, 18 August 2022
Group 3 element (redirect from Group III elements)
masked by the formation of an oxide layer. The first three of them occur naturally, and especially yttrium and lutetium are almost invariably associated...
53 KB (5,847 words) - 07:55, 25 March 2024
Scandium oxide forms scandate salts with alkalis, unlike its higher homologues yttrium oxide and lanthanum oxide (but like lutetium oxide), for example...
6 KB (555 words) - 13:32, 15 July 2023
also forms crystalline hydrates. The compound is poisonous. Dissolving lutetium oxide in nitric acid: L u 2 O 3 + 6 H N O 3 → 90 o C 2 L u ( N O 3 ) 3...
4 KB (391 words) - 01:43, 29 March 2024
be prepared by reacting lutetium oxide and hydrogen selenide at a high temperature: Lu2O3 + 3 H2Se → Lu2Se3 + 3 H2O Lutetium(III) selenide can form orthorhombic...
2 KB (218 words) - 14:39, 21 January 2024
Periodic table (section Valence and oxidation states)
elements (lutetium through mercury) follows, and finally six main-group elements (thallium through radon) complete the period. From lutetium onwards the...
251 KB (27,189 words) - 18:37, 29 May 2024
Lutetium(III) hydroxide is an inorganic compound with the chemical formula Lu(OH)3. Reacting lutetium chloride and alkalis will first produce Lu(OH)2Cl...
2 KB (157 words) - 13:36, 31 December 2022
Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3. Lutetium(III) iodide...
4 KB (359 words) - 02:59, 8 September 2023
Chromium (redirect from Chromium(III))
number of chromium(III) compounds are known, such as chromium(III) nitrate, chromium(III) acetate, and chromium(III) oxide. Chromium(III) can be obtained...
99 KB (11,258 words) - 16:12, 19 May 2024
Latin 21 Lutetia, an asteroid Lutetia, a typeface by Jan van Krimpen Lutetium(III) oxide, a chemical compound A variant of the given name Letitia All pages...
643 bytes (93 words) - 18:34, 7 February 2023
Terbium acetate (redirect from Terbium(III) acetate)
decompose at 220 °C, forming terbium oxide at 650 °C. Manabe, Kazuo; Ogawa, Makoto. Thermal decomposition of terbium(III) acetate tetrahydrate (in Japanese)...
3 KB (116 words) - 17:08, 2 September 2023
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It...
46 KB (14,122 words) - 16:20, 30 May 2024
Lanthanide (redirect from Lanthanide oxide)
lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a...
106 KB (10,221 words) - 15:43, 19 May 2024
Lanthanide trifluoromethanesulfonates (redirect from Lutetium(III) triflate)
Crystal structures of [M(OH2)9] [CF3SO3]3, M = lanthanum, gadolinium, lutetium, or yttrium". Australian Journal of Chemistry. 36 (3): 483–492. doi:10...
8 KB (924 words) - 07:50, 18 October 2023
Gallium (section Oxides and chalcogenides)
most other materials (with the exceptions of quartz, graphite, gallium(III) oxide and PTFE), making it mechanically more difficult to handle even though...
74 KB (8,755 words) - 22:11, 1 June 2024
number. c The correct composition of group 3 is scandium (Sc), yttrium (Y), lutetium (Lu), and lawrencium (Lr), as shown here: this is endorsed by 1988 and...
12 KB (1,570 words) - 22:13, 2 March 2024
Indium (section Other oxidation states)
needed to involve the 5s-electrons. Indium(I) oxide and hydroxide are more basic and indium(III) oxide and hydroxide are more acidic. A number of standard...
47 KB (5,599 words) - 00:36, 15 May 2024
Iron (section Molten oxide electrolysis)
due to its oxide layer. Iron forms various oxide and hydroxide compounds; the most common are iron(II,III) oxide (Fe3O4), and iron(III) oxide (Fe2O3). Iron(II)...
148 KB (16,943 words) - 19:15, 3 June 2024
Isotopes of hafnium and lutetium (along with ytterbium) are also used in isotope geochemistry and geochronological applications, in lutetium-hafnium dating. It...
46 KB (5,394 words) - 10:29, 12 May 2024