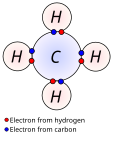
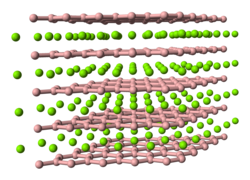
A network solid or covalent network solid (also called atomic crystalline solids or giant covalent structures) is a chemical compound (or element) in which...
4 KB (462 words) - 14:40, 16 May 2025
covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal...
29 KB (3,858 words) - 13:52, 2 June 2025
complex ion Ag(NH3)2+, which has two Ag←N coordinate covalent bonds. In metallic bonding, bonding electrons are delocalized over a lattice of atoms. By...
40 KB (4,878 words) - 16:35, 25 May 2025
chemistry, where hydrogen bonding spans a continuum from weak van der Waals-like interactions to nearly covalent bonding. Hydrogen bonding can occur between separate...
47 KB (5,592 words) - 12:32, 25 May 2025
which forms network covalent solids (sometimes called simply "covalent solids") Ionic bonding, which forms ionic solids Metallic bonding, which forms...
11 KB (1,375 words) - 20:58, 10 February 2025
Covalent adaptable networks (CANs) are a type of polymer material that closely resemble thermosetting polymers (thermosets). However, they are distinguished...
28 KB (3,284 words) - 20:02, 8 February 2025
Smart rubber (section Hydrogen bonding networks)
would depend on covalent bonding and ionic bonding between chains, which is typical of normal rubber. In this case hydrogen bonding can occur simply...
3 KB (328 words) - 18:44, 3 September 2023
Valence electron (category Chemical bonding)
formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing...
24 KB (2,333 words) - 15:26, 27 November 2024
average bond valence is known as the bonding strength of the atom. Since the bonding strength of an atom is the valence expected for a bond formed by...
23 KB (3,337 words) - 01:02, 26 May 2025
Supramolecular chemistry (redirect from Supramolecular network)
intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small...
35 KB (3,883 words) - 07:48, 12 May 2025
scale) that they form covalent bonds in which the electrons are shared almost equally. Ruthenium boride Network covalent bonding Superhard material Gaidar'...
11 KB (1,052 words) - 01:45, 24 May 2025
together. Bonding is a mutual, interactive process, and is different from simple liking. It is the process of nurturing social connection. Bonding typically...
32 KB (4,279 words) - 05:42, 3 June 2025
non-covalent assembly uses adsorbed monolayers to create binding sites for target molecules through hydrogen bonding interactions. Hydrogen bonding is...
27 KB (2,726 words) - 11:18, 3 March 2025
interactions–that is, non-covalent bonds. These non-covalent interactions include van der Waals interactions, hydrogen bonding, Coulomb or ionic interactions...
49 KB (5,691 words) - 17:58, 2 February 2025
them. Vitrimers consist of molecular, covalent networks, which can change their topology by thermally activated bond-exchange reactions. At high temperatures...
17 KB (1,962 words) - 15:59, 31 May 2025
cross-link is a bond or a short sequence of bonds that links one polymer chain to another. These links may take the form of covalent bonds or ionic bonds...
18 KB (1,797 words) - 10:15, 25 May 2025
polymer network (IPN) is a polymer comprising two or more networks which are at least partially interlaced on a polymer scale but not covalently bonded...
16 KB (1,959 words) - 03:10, 29 May 2025
properties. When atomic orbitals overlap during metallic or covalent bonding, they create both bonding and antibonding molecular orbitals of equal capacity,...
252 KB (27,179 words) - 14:30, 25 May 2025
Self-healing hydrogels (section Hydrogen bonding)
attraction forces drive new bond formation through reconstructive covalent dangling side chain or non-covalent hydrogen bonding. These flesh-like properties...
34 KB (3,601 words) - 11:27, 29 June 2024
Chemical compound (section Bonding and forces)
octet. Ionic bonding occurs when valence electrons are completely transferred between elements. Opposite to covalent bonding, this chemical bond creates two...
23 KB (2,644 words) - 08:02, 10 February 2025
there are few commonly mentioned types of non-covalent interactions: ionic bonding, hydrogen bonding, van der Waals forces and hydrophobic interactions...
45 KB (5,112 words) - 17:59, 31 May 2025
Molecular solid (section Hydrogen and halogen bonding)
interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic...
33 KB (2,941 words) - 16:38, 2 June 2025
Molecular imprinting (section Covalent)
compounds that can be used to imprint with template molecules via covalent bonding, such as alcohols, aldehydes and ketones, all of which have high formation...
19 KB (2,212 words) - 16:58, 26 October 2024
Topological polymers (section Polymer network topology)
hydrogen bonding, etc.) between each of the polymer chain. On the other hand, topology also determines the hierarchical structures within a polymer network, from...
20 KB (2,513 words) - 02:47, 2 June 2025
agent is a molecule in water solution that can disrupt the hydrogen bonding network between water molecules (i.e. exerts chaotropic activity). This has...
5 KB (604 words) - 08:43, 17 September 2024
the corresponding chemical species. The bonding between the components is normally weaker than in a covalent bond. The term has also been used with a variety...
12 KB (1,405 words) - 10:00, 27 March 2024
characterised by a double bond between a carbon and nitrogen atom. The term polyimine can also be found occasionally in covalent organic frameworks (COFs)...
8 KB (952 words) - 09:03, 17 March 2025
toxic substances exert their toxicity through some interaction (e.g., covalent bonding, oxidation) with cellular macromolecules like proteins or DNA. This...
4 KB (388 words) - 15:23, 13 January 2024
hydrogen bonding is known to facilitate the formation of chain structures. For example, 4-tricyclanol C10H16O shows catenated hydrogen bonding between...
12 KB (1,464 words) - 13:24, 26 May 2025