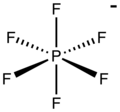
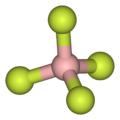
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating...
9 KB (898 words) - 23:02, 4 June 2025
Hexafluorophosphate (category Non-coordinating anions)
[SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion. Hexafluorophosphate...
14 KB (1,222 words) - 10:30, 20 June 2025
Tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (redirect from Kobayashi's anion)
to refer to anions which are unlikely to bind directly to the metal centre of a complex. Hexafluorophosphate is a non-coordinating anion in both senses...
11 KB (1,061 words) - 02:29, 27 May 2025
Bistriflimide (category Non-coordinating anions)
is a non-coordinating anion with the chemical formula [(CF3SO2)2N]−. Its salts are typically referred to as being metal triflimidates. The anion is widely...
6 KB (626 words) - 14:54, 2 August 2023
Perchlorate (category Non-coordinating anions)
weak electron pair donor) and a weak nucleophilic anion, it is also a very weakly coordinating anion. This is why it is often used as a supporting electrolyte...
80 KB (8,898 words) - 04:19, 16 July 2025
Tetrachloroaluminate (redirect from Aluminium tetrachloride anion)
Tetrachloroaluminate [AlCl4]− is an anion formed from aluminium and chlorine. The anion has a tetrahedral shape and is isoelectronic with silicon tetrachloride...
4 KB (185 words) - 08:25, 12 December 2024
in non-polar solvents. The tetrachloroferrate anion, with iron(III) in the center, has tetrahedral geometry. It is useful as a non-coordinating anion comparable...
3 KB (310 words) - 00:22, 21 July 2023
is the lithium salt of the weakly coordinating anion (B(C6F5)4)−. Because of its weakly coordinating abilities, lithium tetrakis(pentafluorophenyl)borate...
7 KB (572 words) - 05:20, 18 July 2025
Nitrate (category Non-coordinating anions)
An example of an insoluble nitrate is bismuth oxynitrate. The nitrate anion is the conjugate base of nitric acid, consisting of one central nitrogen...
38 KB (4,299 words) - 16:06, 20 July 2025
Brookhart's acid (category Non-coordinating anions)
weakly coordinating anion; it was first reported in 2000. An X-ray crystal structure of that compound was obtained, showing the acidic proton coordinated by...
11 KB (965 words) - 21:18, 31 May 2022
cation, [At(C5H5N)2]+, forms ionic compounds with perchlorate (a non-coordinating anion) and with nitrate, [At(C5H5N)2]NO3. This cation exists as a coordination...
82 KB (9,336 words) - 06:00, 21 July 2025
Tetrafluoroborate (category Non-coordinating anions)
conveniently the smallest weakly coordinating anion from the point of view of equivalent weight, often making it the anion of choice for preparing cationic...
9 KB (1,023 words) - 20:37, 23 December 2024
KPF6 is a common laboratory source of the hexafluorophosphate anion, a non-coordinating anion that confers lipophilicity to its salts. These salts are often...
3 KB (177 words) - 15:29, 19 December 2024
triflates. The triflide anion has also been employed as the anionic component of ionic liquids. Bistriflimide Non-coordinating anion Barrett, A. G. M.; Braddock...
5 KB (372 words) - 22:27, 16 October 2024
(2005). "New ionic liquids with tris(perfluoroalkyl)trifluorophosphate (FAP) anions". Journal of Fluorine Chemistry. 126 (8): 1150–1159. Bibcode:2005JFluC.126...
44 KB (1,929 words) - 09:37, 8 July 2025
Its conjugate base, hexafluorophosphate ([PF6]−), is a useful non-coordinating anion. It is often used in lithium-ion batteries, where besides providing...
6 KB (459 words) - 03:46, 23 May 2025
compounds have long been exploited as both stable conjugate acids of non-coordinating anions (SbF− 6 and Sb 2F− 11), and strong Lewis acid counterparts of well-known...
31 KB (3,067 words) - 21:55, 3 July 2025
replacing halides ligands with perchlorate, which is a weakly or non-coordinating anion. The use of silver perchlorate in chemical synthesis has declined...
5 KB (337 words) - 23:09, 1 February 2025
Tetraphenylborate (category Anions)
Triphenylborane BARF and other fluorinated derivatives are used as non-coordinating anions. Utsumi, Kozo; Packer, Lester (1967). "Uncoupling of energy transfer...
2 KB (76 words) - 21:16, 19 February 2024
N-oxoammonium salts derived from TMP: The oxoammonium salts with non-coordinating anions are used (such as tetrafluoroborate, perchlorate, hexafluorophosphate...
7 KB (684 words) - 06:30, 23 May 2025
acetonitrile or acetone. Copper(I) sources can vary, though sources with non-coordinating anions like triflate, tetrafluoroborate, and hexafluorophosphate are preferred...
20 KB (2,216 words) - 18:34, 18 July 2025
BF3·O(CH2CH3)2 Tetrafluoroborate salts are commonly employed as non-coordinating anions. The adduct with diethyl ether, boron trifluoride diethyl etherate...
20 KB (1,911 words) - 07:49, 19 July 2025
AgX(s) where X− = Cl− , Br− , or I− . Other silver salts with non-coordinating anions, namely silver tetrafluoroborate and silver hexafluorophosphate...
24 KB (2,345 words) - 22:54, 25 May 2025
form related fluoride-anions, hexafluorophosphate, hexafluoroarsenate, hexafluoroantimonate, that function as non-coordinating anions. Phosphorus even forms...
34 KB (4,011 words) - 05:12, 2 July 2025
borane is more favorable than homolysis into radical cation and radical anion. However, aside from clear-cut examples, there is considerable dispute as...
10 KB (1,310 words) - 15:12, 21 May 2025
(2007). "Isobutene Polymerization Using [CuII(NCMe)6]2+ with Non-Coordinating Anions as Catalysts". Macromolecular Rapid Communications. 28 (5): 670–675...
19 KB (1,986 words) - 16:20, 20 July 2025
Silveira, Alexandre (3 February 1983). "Hexafluoroarsenate as a Non-Coordinating Anion in Lanthanide Complexes with the Diphenyl Sulphoxide Ligand". Journal...
9 KB (807 words) - 10:58, 10 February 2025
Area 3, lying north of Everglades National Park Weakly coordinating anion, or non-coordinating anion Worst-case circuit analysis WAGR WCA/WCE class WestAir...
4 KB (500 words) - 20:09, 12 May 2025
terbium(III,IV) oxide with perchloric acid. The perchlorates are non-coordinating anions, so this substance can be used as a starting material for forming...
4 KB (340 words) - 17:58, 17 March 2025
cation, [At(C5H5N)2]+, forms ionic compounds with perchlorate (a non-coordinating anion) and with nitrate, [At(C5H5N)2]NO3. This cation exists as a coordination...
21 KB (2,345 words) - 13:20, 15 June 2025