Oxidation with chromium(VI) complexes involves the conversion of alcohols to carbonyl compounds or more highly oxidized products through the action of...
8 KB (1,006 words) - 18:26, 9 June 2025
chromium (III), the oxidation-reduction conversions between the two oxidation states have implications for movement and bioavailability of chromium in...
113 KB (12,320 words) - 05:32, 14 June 2025
Chromium(VI) oxide peroxide is a chemical compound with the chemical formula CrO(O2)2. The name "chromium(VI) oxide peroxide" is also given to a collection...
6 KB (514 words) - 13:50, 2 May 2025
Hexavalent chromium (chromium(VI), Cr(VI), chromium 6) is any chemical compound that contains the element chromium in the +6 oxidation state (thus hexavalent)...
66 KB (7,085 words) - 03:45, 20 May 2025
sigmatotropic reaction as for tertiary alcohols. Oxidation with chromium(VI) complexes Oxoammonium-catalyzed oxidation Other reactions of PCC Killoran, Patrick...
7 KB (684 words) - 06:30, 23 May 2025
within chromium compounds, followed by +2; charges of +1, +4 and +5 for chromium are rare, but do nevertheless occasionally exist. Many Cr(0) complexes are...
14 KB (1,400 words) - 06:14, 26 May 2025
The Sarett oxidation is an organic reaction that oxidizes primary and secondary alcohols to aldehydes and ketones, respectively, using chromium trioxide...
8 KB (841 words) - 03:51, 28 December 2024
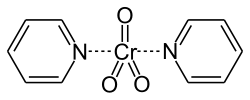
over oxidation to carboxylic acids. Chromium(VI) reagents are commonly used for these oxidations. One family of Cr(VI) reagents employs the complex CrO3(pyridine)2...
15 KB (1,731 words) - 06:45, 9 June 2025
chromium trioxide. The oxidation is very rapid and quite exothermic. Yields are typically high. The reagent is convenient and cheap. However, Cr(VI)...
8 KB (977 words) - 08:03, 13 March 2025
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms are fully ionic. It describes...
47 KB (11,918 words) - 15:23, 12 May 2025
reagent, a complex of chromium(VI) oxide with pyridine in dichloromethane. The mechanism of the Collins oxidation is a relatively simple oxidation process...
5 KB (475 words) - 17:57, 3 January 2025
Chromite (redirect from Chromium ore)
primarily of iron(II) oxide and chromium(III) oxide compounds. It can be represented by the chemical formula of FeCr2O4. It is an oxide mineral belonging...
27 KB (2,955 words) - 10:40, 25 May 2025
Collins reagent (redirect from Dipyridine chromium(VI) oxide)
popularized by J. C. Collins several years later. Sarett oxidation Oxidation with chromium(VI)-amine complexes Collins reagent can be used as an alternative to...
5 KB (474 words) - 22:58, 15 June 2025
bis(benzene)chromium. The compound reacts with carboxylic acids to give chromium(II) carboxylates, such as chromium(II) acetate. Oxidation affords [Cr(C6H6)2]+...
17 KB (1,555 words) - 18:26, 22 May 2025
Chromic acid (category Articles with short description)
trioxide is the anhydride. Chromic acid features chromium in an oxidation state of +6 (and a valence of VI or 6). It is a strong and corrosive oxidizing...
15 KB (1,370 words) - 13:35, 22 June 2025
Chromate and dichromate (category Articles with short description)
contain the dichromate anion, Cr2O2−7. They are oxyanions of chromium in the +6 oxidation state and are moderately strong oxidizing agents. In an aqueous...
13 KB (1,261 words) - 18:39, 24 May 2025
Manganese (category Articles with Encyclopædia Britannica links)
Common oxidation states of manganese are +2, +3, +4, +6, and +7, although all oxidation states from −3 to +7 have been observed. Manganese in oxidation state...
91 KB (10,177 words) - 00:09, 21 June 2025
Chromium(II) acetate hydrate, also known as chromous acetate, is the coordination compound with the formula Cr2(CH3CO2)4(H2O)2. This formula is commonly...
11 KB (991 words) - 14:12, 24 March 2025
hydrochloric acid and aqueous methanol. Slow reaction rates are common with chromium(III) complexes. The low reactivity of the d3 Cr3+ ion can be explained using...
15 KB (1,354 words) - 22:27, 17 March 2025
Transition metal complexes of thiocyanate describes coordination complexes containing one or more thiocyanate (SCN−) ligands. The topic also includes...
16 KB (1,766 words) - 17:09, 28 April 2025
Aldehyde (redirect from Corey-Gilman-Ganem oxidation)
often consumed stoichiometrically. chromium(VI) reagents are popular. Oxidation can be achieved by heating the alcohol with an acidified solution of potassium...
30 KB (3,112 words) - 13:18, 1 June 2025
Chrome plating (redirect from Chromium plating)
to prevent the oxidation of trivalent chromium to the anodes. A sulfate-based bath that uses lead anodes surrounded by boxes filled with sulfuric acid...
28 KB (3,362 words) - 03:10, 16 June 2025
carbynes (RC:::CrLn) Chromium(III) complexes RCrL5. Complexes of chromium carbonyl anion and cation (e.g Na4Cr(CO)4). Chromium catalysts are important...
11 KB (1,312 words) - 23:18, 12 May 2024
Pyridinium chlorochromate (category Articles with changed InChI identifier)
is its toxicity, which it shares with other hexavalent chromium compounds. Oxidation with chromium(VI)-amine complexes Piancatelli, G.; Luzzio, F. A. (2007)...
10 KB (780 words) - 19:45, 11 April 2025
give Fischer carbene complexes: Treatment of chromium hexacarbonyl with sodium cyclopentadienide gives Na+[Cr(CO)3(C5H5)]−. Oxidation of this salt affords...
18 KB (1,557 words) - 21:05, 22 May 2025
It is common in academic laboratories for the synthesis of chromium coordination complexes. The relatively complicated formula - [Cr(H2O)6](NO3)3•3H2O...
4 KB (262 words) - 17:15, 2 February 2025
Tungsten (category Articles with short description)
mid-level oxidation states are often associated with metal clusters, and very low oxidation states are typically associated with CO complexes. The chemistries...
82 KB (9,428 words) - 05:32, 14 June 2025
Potassium dichromate (redirect from Potassium dichromate(VI))
compound with the formula K2Cr2O7. An orange solid, it is used in diverse laboratory and industrial applications. As with all hexavalent chromium compounds...
18 KB (1,786 words) - 15:49, 4 June 2025
dithiocarbamate complexes are coordination complexes containing one or more dithiocarbamate ligand, which are typically abbreviated R2dtc−. Many complexes are known...
12 KB (1,213 words) - 09:21, 23 June 2025