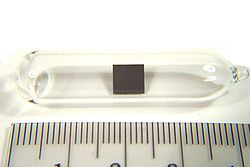
Tin(II) oxalate is an inorganic compound, a salt of tin and oxalic acid with the chemical formula SnC 2O 4. The compound looks like colorless crystals...
4 KB (369 words) - 20:52, 18 July 2025
ammonia on a tin(II) salt. SnO may be prepared as a pure substance in the laboratory, by controlled heating of tin(II) oxalate (stannous oxalate) in the absence...
10 KB (917 words) - 23:26, 22 July 2025
Sn(BF4)2 tin(II) fluoroborate 13814–97–6 SnBr2 tin(II) bromide 10031–24–0 SnBr4 tin(IV) bromide 7789–67–5 SnC2O4 tin(II) oxalate 814–94–8 SnCl2 tin(II) chloride...
139 KB (120 words) - 17:15, 19 May 2025
137–146. doi:10.1080/00958979009409182. "Properties of substance: mercury(II) oxalate Group of substances". Chemister. Retrieved 13 September 2022. Burkhart...
35 KB (1,082 words) - 10:57, 23 July 2025
An oxalate fluoride is a mixed anion compound contains both oxalate and fluoride anions. Many of these compounds have high birefringence. With nonmetals...
52 KB (2,464 words) - 14:11, 28 July 2025
Tin(II) bromide – SnBr2 Tin(II) chloride (stannous chloride) – SnCl2 Tin(II) fluoride – SnF2 Tin(II) hydroxide – Sn(OH)2 Tin(II) iodide – SnI2 Tin(II)...
122 KB (9,014 words) - 06:41, 30 June 2025
(perovskite, schoenfliesite: IMA1980-028) 4.FC.10 [104] [105] [106] (IUPAC: iron(II) tin(IV) hexahydroxide) Natisite (IMA1974-035) 9.AG.40a [107] [108] [109] Natrite...
39 KB (2,824 words) - 17:47, 9 February 2024
Tm(NO3)3 212 Tin(II) bromide SnBr2 85 Tin(II) chloride SnCl2 84 Tin(II) fluoride SnF2 30 Tin(II) iodide SnI2 0.99 1.17 1.42 2.11 3.04 3.58 4.2 Tin(II) sulfate...
84 KB (284 words) - 04:33, 30 June 2025
magnesium heptafluoroaluminate) Weddellite (oxalate: 1936) 10.AB.40 [107] [108] [109] (IUPAC: calcium oxalate dihydrate) Weeksite (IMA1962 s.p., 1960) 9...
33 KB (2,391 words) - 17:36, 9 February 2024
Ammonium hexachlorostannate (redirect from Ammonium tin chloride)
chemical compound with the chemical formula (NH4)2SnCl6. A reaction of pure tin tetrachloride with ammonium chloride: SnCl4 + 2NH4Cl → (NH4)2SnCl6 The compound...
3 KB (127 words) - 05:43, 23 January 2025
is referred to as Fe(III), FeIII or Fe III (Fe I for a neutral Fe atom, Fe II for a singly ionized Fe ion). The Roman numeral designates the formal oxidation...
31 KB (3,198 words) - 16:00, 28 May 2025
pentahydrate was the first known example of coordination number 11, the oxalate tetrahydrate has coordination number 10, and the borohydride (first prepared...
145 KB (16,401 words) - 20:37, 26 July 2025
Anodizing (section Sulfuric acid (Type II & III))
fading. Black dyes and gold produced by inorganic means (ferric ammonium oxalate) are more lightfast. Dyed anodizing is usually sealed to reduce or eliminate...
36 KB (4,556 words) - 07:33, 23 July 2025
(IMA1973-055) 10.CA.40 [95] [96] [97] (IUPAC: uric acid) Uroxite (hydrous uranyl oxalate: IMA2018-100) 10.AB. [98] [no] [no] Ursilite (Y: 1957) 9.AK.35 [99] [100]...
37 KB (2,568 words) - 17:45, 9 February 2024
boulangerite. Falottaite (IMA2013-044) 10.AB. [30] [no] [no] (IUPAC: manganese oxalate trihydrate) Falsterite (IMA2011-061) 8.0 [31] [no] [no] Famatinite (stannite:...
64 KB (4,720 words) - 17:47, 9 February 2024
mechanism of substitution of the quadruply bonded molybdenum(II) aqua dimer with thiocyanate and oxalate". Inorganic Chemistry. 22 (22): 3315–3333. doi:10.1021/ic00164a027...
71 KB (7,653 words) - 02:50, 10 July 2025
9.AE.15 [1,138] [1,139] [1,140] (IUPAC: sodium diberyllium dimanganese(II) tin trisilicate dodecaoxy hydroxyl) Svornostite (IMA2014-078) 7.0 [1,141] [no]...
103 KB (6,878 words) - 17:47, 9 February 2024
photographic processes. The dihydrate of iron(II) oxalate has a polymeric structure with co-planar oxalate ions bridging between iron centres with the water...
150 KB (16,945 words) - 13:04, 14 July 2025
arsenate di(hydroxoarsenate)) Caoxite (oxalate: IMA1996-012) 10.AB.50 [174] [175] [176] (IUPAC: calcium oxalate trihydrate) Capgaronnite (IMA1990-011)...
103 KB (6,708 words) - 17:15, 19 June 2024
the solution is treated with ammonium oxalate to convert rare earths into their insoluble oxalates. The oxalates are converted to oxides by annealing....
33 KB (3,980 words) - 17:34, 26 July 2025
compounds. For example, trivalent americium forms insoluble fluoride, oxalate, iodate, hydroxide, phosphate and other salts. Compounds of americium in...
77 KB (9,327 words) - 13:31, 26 July 2025
[15] (IUPAC: dilead tin indium heptasulfa bismuthide) Abswurmbachite (braunite: IMA1990-007) 9.AG.05 [16] [17] [18] (IUPAC: copper(II) hexamanganese(III)...
73 KB (4,972 words) - 12:37, 5 December 2024
(Cr2O2−7) chromate (CrO2−4) peroxide (O2−2) superoxide (O−2) oxalate (C2O2−4) hydrogen oxalate (HC2O−4) The formula Na2SO3 denotes that the cation is sodium...
26 KB (3,077 words) - 15:37, 1 May 2025
pressure. Francium nitrate, sulfate, hydroxide, carbonate, acetate, and oxalate, are all soluble in water, while the iodate, picrate, tartrate, chloroplatinate...
38 KB (4,281 words) - 04:25, 2 June 2025
prevent afterglow in matches, in purifying sugar; as a flux for soldering tin, copper, zinc and brass; and to control precipitation of alkali-soluble and...
7 KB (540 words) - 22:17, 29 October 2024
Burai L, Riedel M (2004). "Revising the Mechanism of the Permanganate/Oxalate Reaction". J. Phys. Chem. A. 108 (50): 11026–11031. Bibcode:2004JPCA..10811026K...
40 KB (4,499 words) - 10:03, 18 July 2025
green system for recovery of REEs from coal fly ash by using citrate and oxalate who are strong organic ligand and capable of complexing or precipitating...
160 KB (17,102 words) - 17:38, 19 July 2025
amide, and silver fulminate, as well as silver acetylide, silver oxalate, and silver(II) oxide. They can explode on heating, force, drying, illumination...
95 KB (11,424 words) - 15:18, 8 July 2025
beings (biogenic), such as tungsten carbide, urinary calculi, calcium oxalate crystals in plant tissues, and seashells. However, substances with such...
111 KB (13,149 words) - 03:20, 29 June 2025
is also used as an additive in tin-nickel plating processes as the fluoride ion acts as a complexing agent with the tin, allowing for greater control over...
10 KB (783 words) - 19:47, 30 June 2025