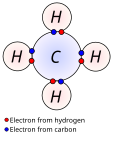
main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal, a valence electron can also be in an inner...
24 KB (2,333 words) - 15:26, 27 November 2024
VSEPR theory (redirect from Valence shell electron pair repulsion)
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,059 words) - 16:52, 29 March 2025
has a valence of 4; in ammonia, nitrogen has a valence of 3; in water, oxygen has a valence of 2; and in hydrogen chloride, chlorine has a valence of 1...
40 KB (2,914 words) - 12:59, 11 January 2025
conductivity of the solid. In nonmetals, the valence band is the highest range of electron energies in which electrons are normally present at absolute zero...
6 KB (701 words) - 07:20, 8 April 2025
Lewis structure (redirect from Electron Dot Structure)
the need for electron counting: the atoms are drawn showing the valence electrons; bonds are then formed by pairing up valence electrons of the atoms...
16 KB (2,159 words) - 08:48, 30 April 2025
Covalent bond (redirect from One-electron bond)
the atoms share "valence", such as is discussed in valence bond theory. In the molecule H 2, the hydrogen atoms share the two electrons via covalent bonding...
29 KB (3,846 words) - 22:53, 13 April 2025
In chemistry, electron counting is a formalism for assigning a number of valence electrons to individual atoms in a molecule. It is used for classifying...
14 KB (1,629 words) - 15:35, 19 January 2025
Core electrons are the electrons in an atom that are not valence electrons and do not participate as directly in chemical bonding. The nucleus and the...
10 KB (1,398 words) - 16:01, 5 May 2025
molecular geometries. Valence bond theory complements molecular orbital theory, which does not adhere to the valence bond idea that electron pairs are localized...
15 KB (1,887 words) - 16:20, 15 March 2025
organometallic compounds. The rule is based on the fact that the valence orbitals in the electron configuration of transition metals consist of five (n−1)d orbitals...
17 KB (1,923 words) - 10:21, 9 April 2025
the valence shell of the atom; a group 17 atom releases more energy than a group 1 atom on gaining an electron because it obtains a filled valence shell...
22 KB (1,468 words) - 11:02, 24 April 2025
Radical (chemistry) (redirect from Single electron transfer)
molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many...
41 KB (4,606 words) - 05:39, 5 May 2025
other electron state in the valence band. A hole near the top of the valence band moves the same way as an electron near the top of the valence band would...
15 KB (1,956 words) - 08:12, 24 April 2025
Formal charge (redirect from Valence charge)
the number of valence electrons of the neutral atom in isolation (in its ground state); L is the number of non-bonding valence electrons assigned to this...
9 KB (1,050 words) - 02:14, 19 September 2024
and the "rule of eight", which began to distinguish between valence and valence electrons. In 1919, Irving Langmuir refined these concepts further and...
23 KB (2,880 words) - 15:13, 25 April 2025
Free electron in physics may refer to: Electron, as a free particle Solvated electron Charge carrier, as carriers of electric charge Valence electron, as...
778 bytes (139 words) - 12:54, 17 January 2021
systems, this was the Hückel method proposed by Erich Hückel. For all valence electron systems, the extended Hückel method was proposed by Roald Hoffmann...
12 KB (1,419 words) - 06:03, 22 August 2024
an open shell is a valence shell which is not completely filled with electrons or that has not given all of its valence electrons through chemical bonds...
60 KB (6,206 words) - 11:54, 27 April 2025
Lone pair (redirect from Free electron pair)
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bond and is sometimes called an unshared...
23 KB (2,957 words) - 08:07, 16 March 2025
octet rule because they have too few valence electrons and species that happen to follow the octet rule but have electron-acceptor properties, forming donor-acceptor...
4 KB (411 words) - 22:54, 13 April 2025
Periodic table (section Valence and oxidation states)
both valence electron count and valence orbital type. As chemical reactions involve the valence electrons, elements with similar outer electron configurations...
251 KB (27,136 words) - 13:25, 25 April 2025
Aufbau principle (redirect from Principles in distribution of electrons)
many-electron quantum-mechanical system. The valence d-subshell "borrows" one electron (in the case of palladium two electrons) from the valence s-subshell...
28 KB (3,097 words) - 02:28, 13 April 2025
affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity...
35 KB (4,372 words) - 14:38, 30 April 2025
the paramagnetic nature of O2, which valence bond theory cannot explain. In molecular orbital theory, electrons in a molecule are not assigned to individual...
28 KB (3,701 words) - 14:57, 25 April 2025
resonance hybrid (or hybrid structure) in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed...
42 KB (5,100 words) - 06:18, 24 March 2025
Periodic trends (section Electron affinity)
increases when we go down a group. This is because in periods, the valence electrons are in the same outermost shell. The atomic number increases within...
19 KB (2,130 words) - 01:00, 31 January 2025
Electronic band structure (redirect from Electron band)
however, the bands are neither electron-like nor hole-like, and often just called "valence band" as they are made of valence orbitals. The band gaps in a...
37 KB (4,835 words) - 08:39, 9 December 2024
Ionization energy (redirect from Electron binding energy)
is due to the fact that gadolinium valence d-subshell borrows 1 electron from the valence f-subshell. Now the valence subshell is the d-subshell, and due...
51 KB (5,833 words) - 19:31, 7 February 2025
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...
14 KB (1,701 words) - 03:27, 4 October 2023
lone pair of valence electrons. They also fill the core levels of an atom. Because the spins are paired, the magnetic moment of the electrons cancel one...
3 KB (314 words) - 08:42, 25 April 2025