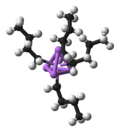
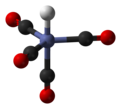
The 18-electron rule is a chemical rule of thumb used primarily for predicting and rationalizing formulas for stable transition metal complexes, especially...
17 KB (1,923 words) - 10:21, 9 April 2025
octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in...
23 KB (2,861 words) - 21:13, 19 June 2025
and oxygen, 18-electron rule in inorganic chemistry and organometallic chemistry of transition metals, Hückel's rule for the π-electrons of aromatic compounds...
14 KB (1,629 words) - 14:30, 26 May 2025
are 18 one-sided pentominoes. In the classification of finite simple groups, there are 18 infinite families of groups. The 18-electron rule is a rule of...
6 KB (670 words) - 16:51, 25 June 2025
shell diagrams. Periodic table (electron configurations) Electron counting 18-electron rule Core charge Re: Why do electron shells have set limits ? madsci...
28 KB (2,765 words) - 11:55, 25 April 2025
This tendency is called the 18-electron rule, because each bonded atom has 18 valence electrons including shared electrons. The heavy group 2 elements...
24 KB (2,333 words) - 14:43, 11 June 2025
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure)...
60 KB (6,208 words) - 21:30, 15 June 2025
Tolman's rule states that, in certain chemical reactions, the steps involve exclusively intermediates of 18- and 16 electron configuration. The rule is an...
2 KB (157 words) - 02:27, 5 June 2025
Aufbau principle (redirect from Principles in distribution of electrons)
'building-up principle'), also called the Aufbau rule, states that in the ground state of an atom or ion, electrons first fill subshells of the lowest available...
28 KB (3,099 words) - 19:02, 17 June 2025
Most species with the formula Mx(CO)y follow the 18-electron rule, whereas V(CO)6 has 17 valence electrons. According to the original synthesis by Calderazzo...
5 KB (431 words) - 12:44, 21 July 2024
enforced by the 18-electron rule, since CpFe(CO)2(η1-C3H5) is already an 18-electron complex, while an η3-allyl ligand would result in an electron count of 20...
13 KB (1,500 words) - 21:37, 23 May 2025
share their electrons. Apparent violations of the 18-electron rule sometimes are explicable in compounds with unusual hapticities: The 18-VE complex...
13 KB (1,316 words) - 13:02, 22 June 2025
are used below. As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. However there are numerous exceptions;...
58 KB (1,165 words) - 08:47, 28 May 2025
In solid-state physics, the electron mobility characterizes how quickly an electron can move through a metal or semiconductor when pushed or pulled by...
52 KB (7,204 words) - 22:26, 22 May 2025
are the five d, one s and three p orbitals with the corresponding 18-electron rule, spxdy hybridisation is used to model the shape of these molecules...
33 KB (3,204 words) - 20:53, 19 May 2025
available. The formulae of many metal carbonyls can be inferred from the 18-electron rule. Group 2 elements calcium, strontium, and barium can all form octacarbonyl...
71 KB (8,030 words) - 18:18, 28 May 2025
be found using the 18-electron rule, saying that the valence shells of a transition metal will collectively accommodate 18 electrons, whereas the symmetry...
66 KB (8,048 words) - 01:14, 16 June 2025
creating a very stable complex, which satisfies the 18-electron rule. The cis-labilization of 18 e− complexes suggests that dissociation of ligand X in...
9 KB (1,053 words) - 14:45, 17 November 2024
bonding in transition metal carbonyl complexes which abide by the 18-electron rule, and others arguing the molecule more accurately contains ionic bonds...
22 KB (2,573 words) - 23:22, 22 May 2025
formula conforms to the 18-electron rule. The molecule is tetrahedral, with four carbonyl (carbon monoxide) ligands. Electron diffraction studies have...
18 KB (1,637 words) - 02:23, 7 April 2025
reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy. Many...
41 KB (4,062 words) - 20:21, 19 June 2025
From the perspective of the 18-electron rule, the four ligands each provides two electrons, for a total of 16-electrons. As such the compound is coordinatively...
13 KB (1,209 words) - 15:19, 22 May 2025
in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities...
31 KB (3,170 words) - 09:43, 24 June 2025
orbital of the ligand. Bridging carbonyl Dewar–Chatt–Duncanson model 18-electron rule Ligand field theory Pi-donor ligands Miessler, Gary L.; Tarr, Donald...
11 KB (1,249 words) - 20:05, 23 May 2025
ISBN 978-0321811059. Rasmussen, Seth C. (5 March 2015). "The 18-electron rule and electron counting in transition metal compounds: theory and application"...
35 KB (3,356 words) - 09:12, 24 June 2025
rule, the 18-electron rule, and Hückel's 4n + 2 pi-electron rule are proven to be useful in predicting the molecular stability. Wade's rules were formulated...
14 KB (1,581 words) - 12:51, 25 September 2023
generally be ruled out if the original metal complex fulfilled the 18-electron rule because the two metal–oxygen bonds would exceed the 18 electron rule. The...
9 KB (1,023 words) - 12:12, 9 October 2024
(oxidation state zero) and [Fe(CO) 4]2− (oxidation state −2) in which the 18-electron rule is obeyed. These complexes are also covalent. Ionic compounds are mostly...
40 KB (4,499 words) - 04:34, 21 June 2025
ions. The s, p, and d orbitals of the metal can accommodate 18 electrons (see 18-Electron rule). The maximum coordination number for a certain metal is thus...
57 KB (5,545 words) - 18:58, 12 June 2025
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such...
20 KB (2,190 words) - 16:15, 15 October 2024