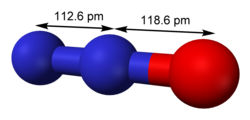
Bismuth(III) oxide is a compound of bismuth, and a common starting point for bismuth chemistry. It is found naturally as the mineral bismite (monoclinic)...
19 KB (2,027 words) - 21:57, 23 May 2025
This page provides supplementary chemical data on bismuth(III) oxide. MSDS from Fischer Scientific This box: view edit Except where noted otherwise...
4 KB (34 words) - 11:53, 12 April 2023
group 15 siblings arsenic and antimony. Elemental bismuth occurs naturally, and its sulfide and oxide forms are important commercial ores. The free element...
67 KB (7,653 words) - 10:19, 22 June 2025
Lanthanum(III) oxide, also known as lanthana, chemical formula La2O3, is an inorganic compound containing the rare earth element lanthanum and oxygen....
9 KB (738 words) - 19:34, 11 April 2025
have been developed. In the solid state reaction method bismuth oxide (Bi2O3) and iron oxide (Fe2O3) in a 1:1 mole ratio are mixed with a mortar or by...
17 KB (1,787 words) - 04:40, 28 June 2025
Bismuth oxynitrate is the name applied to a number of compounds that contain Bi3+, nitrate ions and oxide ions and which can be considered as compounds...
13 KB (1,110 words) - 19:04, 23 December 2024
Antimony (redirect from Antimony(III))
and bismuth. Springer. ISBN 978-0-7514-0389-3. Wiberg and Holleman, p. 757 Long, G.; Stevens, J. G.; Bowen, L. H.; Ruby, S. L. (1969). "The oxidation number...
59 KB (7,026 words) - 23:29, 22 June 2025
H2SO4 → 2 N2O + 2 CO2 + (NH4)2SO4 + 2 H2O Direct oxidation of ammonia with a manganese dioxide-bismuth oxide catalyst has been reported: cf. Ostwald process...
96 KB (9,896 words) - 13:36, 15 July 2025
Hotop, H. (1999). "Binding Energies in Atomic Negative Ions: III". J. Phys. Chem. Ref. Data. 28 (6): 1511. Bibcode:1999JPCRD..28.1511A. doi:10.1063/1.556047...
59 KB (4,157 words) - 11:39, 12 July 2025
Antimony trioxide (redirect from Antimony(III) oxide)
Antimony(III) oxide is the inorganic compound with the formula Sb2O3. It is the most important commercial compound of antimony. It is found in nature as...
12 KB (1,091 words) - 21:23, 15 July 2025
List of semiconductor materials (redirect from III-V semiconductor)
antimony, bismuth). The range of possible formulae is quite broad because these elements can form binary (two elements, e.g. gallium(III) arsenide (GaAs))...
54 KB (2,525 words) - 16:50, 24 May 2025
SOHIO method, which is based on the catalytic oxidation of propylene in the presence of ammonia and bismuth phosphomolybdate. However, until 1960 a key...
108 KB (11,635 words) - 00:53, 6 July 2025
Chromium (redirect from Chromium(III))
number of chromium(III) compounds are known, such as chromium(III) nitrate, chromium(III) acetate, and chromium(III) oxide. Chromium(III) can be obtained...
113 KB (12,320 words) - 22:12, 11 July 2025
Lead(II) oxide, also called lead monoxide, is the inorganic compound with the molecular formula PbO. It occurs in two polymorphs: litharge having a tetragonal...
14 KB (1,552 words) - 13:17, 14 July 2025
Moscovium (redirect from Eka-Bismuth)
as McOCl and McOBr, again analogous to bismuth. Both moscovium(I) and moscovium(III) should be common oxidation states and their relative stability should...
49 KB (8,547 words) - 12:21, 15 May 2025
Iron (category Pages using the Phonos extension)
due to its oxide layer. Iron forms various oxide and hydroxide compounds; the most common are iron(II,III) oxide (Fe3O4), and iron(III) oxide (Fe2O3). Iron(II)...
150 KB (16,945 words) - 13:04, 14 July 2025
Moscovium(I) oxide (Mc2O) should be quite basic, like that of thallium, while moscovium(III) oxide (Mc2O3) should be amphoteric, like that of bismuth. Selenium...
125 KB (15,308 words) - 22:06, 30 April 2025
Periodic table (category Wikipedia indefinitely move-protected pages)
in odd periods of group 15 readily reach the +5 oxidation state, whereas nitrogen, arsenic, and bismuth in even periods prefer to stay at +3. A similar...
251 KB (27,123 words) - 20:02, 11 July 2025
Uranium dioxide (redirect from Uranium(IV) oxide)
Uranium dioxide or uranium(IV) oxide (UO2), also known as urania or uranous oxide, is an oxide of uranium, and is a black, radioactive, crystalline powder...
27 KB (2,628 words) - 20:33, 20 May 2025
Tin (category Wikipedia articles needing page number citations from June 2021)
of small amounts of bismuth, antimony, lead, and silver present as impurities. Alloying elements such as copper, antimony, bismuth, cadmium, and silver...
83 KB (9,198 words) - 19:36, 2 July 2025
Indium (category Chembox having GHS data)
needed to involve the 5s-electrons. Indium(I) oxide and hydroxide are more basic and indium(III) oxide and hydroxide are more acidic. A number of standard...
48 KB (5,678 words) - 04:17, 9 July 2025
Gallium (category Wikipedia articles needing page number citations from April 2024)
most other materials (with the exceptions of quartz, graphite, gallium(III) oxide and PTFE),: 221 making it mechanically more difficult to handle even...
75 KB (8,964 words) - 12:58, 15 July 2025
Ferrite (magnet) (category Wikipedia articles needing page number citations from January 2014)
Fe(II)-Fe(III)2O4. Above 585 °C Fe(II)-Fe(III)2O4 transforms into the non-magnetic gamma phase. Fe(II)-Fe(III)2O4 is commonly seen as the black iron(II) oxide coating...
34 KB (3,774 words) - 22:13, 6 June 2025
Copper (redirect from Copper(III))
superconductors. Yttrium barium copper oxide (YBa2Cu3O7) consists of both Cu(II) and Cu(III) centres. Like oxide, fluoride is a highly basic anion and...
124 KB (13,475 words) - 15:29, 15 July 2025
Uranium (category Pages using the Phonos extension)
(yellow), for U(III), U(IV), U(V), and U(VI), respectively. A few solid and semi-metallic compounds such as UO and US exist for the formal oxidation state uranium(II)...
111 KB (12,425 words) - 18:55, 16 July 2025
Cobalt (redirect from Cobalt(III))
the blue cobalt(II,III) oxide (Co3O4), which has a spinel structure. Black cobalt(III) oxide (Co2O3) is also known. Cobalt oxides are antiferromagnetic...
123 KB (12,634 words) - 10:48, 7 July 2025
Tungsten (category Pages using the Phonos extension)
tungsten(III), for example, contrasts with the pervasiveness of the chromium(III) compounds. The highest oxidation state is seen in tungsten(VI) oxide (WO3)...
83 KB (9,442 words) - 14:53, 12 July 2025
Metal (category Wikipedia indefinitely move-protected pages)
Antimony, showing its brilliant lustre Bismuth in crystalline form, with a very thin oxidation layer, and a 1 cm3 bismuth cube The first systematic text on...
103 KB (12,014 words) - 16:58, 23 June 2025
Terbium(III) bromide (TbBr3) is a crystalline chemical compound. Terbiun(III) bromide can be produced by heating terbium metal or terbium(III) oxide with...
4 KB (253 words) - 19:49, 26 May 2025
H. L.; Marshall, A. L. (1940). "Vapor Pressures of Nickel and of Nickel Oxide". Journal of the American Chemical Society. 62 (6). American Chemical Society...
118 KB (1,365 words) - 16:47, 12 November 2023