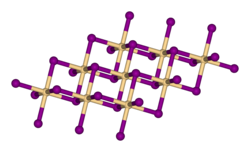
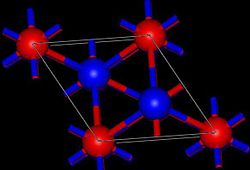
Cadmium iodide is an inorganic compound with the formula CdI2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate. It...
6 KB (435 words) - 00:12, 11 March 2025
Anhydrous cadmium chloride forms a layered structure consisting of octahedral Cd2+ centers linked with chloride ligands. Cadmium iodide, CdI2, has a...
13 KB (791 words) - 14:35, 12 April 2025
concentrations, the British Pharmaceutical Codex from 1907 states that cadmium iodide was used as a medication to treat "enlarged joints, scrofulous glands...
67 KB (7,600 words) - 00:10, 17 July 2025
iron(II) iodide. Crystalline hydrates precipitate from these solutions.[clarification needed] Iron(II) iodide adopts the same crystal structure as cadmium iodide...
5 KB (477 words) - 14:39, 9 May 2024
bromide – ZnBr2 Cadmium arsenide – Cd3As2 Cadmium bromide – CdBr2 Cadmium chloride – CdCl2 Cadmium fluoride – CdF2 Cadmium iodide – CdI2 Cadmium nitrate – Cd(NO3)2...
122 KB (9,014 words) - 06:41, 30 June 2025
Mercury(II) iodide is a chemical compound with the molecular formula HgI2. It is typically produced synthetically but can also be found in nature as the...
6 KB (377 words) - 23:41, 19 June 2025
Zinc iodide is the inorganic compound with the formula ZnI2. It exists both in anhydrous form and as a dihydrate. Both are white and readily absorb water...
8 KB (631 words) - 11:31, 24 May 2025
cadmium chloride 10108–64–2 CdCrO4 cadmium chromate 14312–00–6 CdF2 cadmium fluoride 7790–79–6 Cd(IO3)2 cadmium iodate 7790–81–0 CdI2 cadmium iodide 7790–80–9...
139 KB (120 words) - 17:15, 19 May 2025
structure is identical to that of Mg(OH)2 (brucite structure); i.e., the cadmium iodide motif. Strong hydrogen bonds exist between the layers. Calcium hydroxide...
23 KB (2,239 words) - 23:03, 20 July 2025
Vanadium(II) iodide is the inorganic compound with the formula VI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring octahedral...
3 KB (269 words) - 03:53, 6 October 2023
occupied) 0.4142 cadmium chloride 5.61 cadmium iodide 4.71 MX3 6:2 one-third octahedral 0.4142 rhodium(III) bromide 6.67 bismuth iodide 8.26 M2X3 6:4 two-thirds...
63 KB (6,987 words) - 00:09, 20 July 2025
quantity (0.8 equiv) of cadmium iodide (CdI2) is needed to promote the reaction. Alternatively, the use of cuprous bromide and zinc iodide sequentially as catalysts...
11 KB (1,302 words) - 00:02, 26 June 2025
Cadmium hydroxide is an inorganic compound with the formula Cd(OH)2. It is a white crystalline ionic compound that is a key component of nickel–cadmium...
5 KB (435 words) - 15:56, 11 March 2025
Cadmium acetate is the chemical compound with the formula Cd(O2CCH3)2(H2O)2. The compound is marketed both as the anhydrous form and as a dihydrate, both...
6 KB (310 words) - 15:10, 6 May 2025
Cadmium bromide is the inorganic compound with the formula CdBr2. It is a white hygroscopic solid. It also can be obtained as a mono- and tetrahydrate...
4 KB (162 words) - 06:52, 20 July 2025
Staining (section Propidium iodide)
chemicals used in electron microscopy staining include: ammonium molybdate, cadmium iodide, carbohydrazide, ferric chloride, hexamine, indium trichloride, lanthanum(III)...
47 KB (5,392 words) - 19:09, 30 July 2025
very toxic, along with other cadmium and cyanide compounds. Cadmium cyanide is prepared commercially by treating cadmium hydroxide with hydrogen cyanide:...
5 KB (341 words) - 14:59, 24 March 2025
Cadmium fluoride (CdF2) is a mostly water-insoluble source of cadmium used in oxygen-sensitive applications, such as the production of metallic alloys...
8 KB (628 words) - 00:17, 11 March 2025
Radium iodide is an inorganic compound of radium and iodine with the chemical formula RaI2. It is the radium salt of hydrogen iodide, consisting of radium...
3 KB (147 words) - 21:17, 12 July 2025
eriochalcite, respectively. Anhydrous copper(II) chloride adopts a distorted cadmium iodide structure. In this structure, the copper centers are octahedral. Most...
30 KB (2,744 words) - 10:51, 23 July 2025
and slightly soluble in chloroform and carbon tetrachloride. It has a cadmium iodide structure with lattice parameters a = 413 pm and c = 679 pm. It disproportionates...
4 KB (346 words) - 04:23, 20 September 2023
Titanium(II) iodide is the inorganic compound with the formula TiI2. It is a black micaceous solid. It adopts the cadmium iodide structure, featuring...
1 KB (87 words) - 15:02, 18 December 2022
oxide reacts with acids to make thallium(I) salts. Tl2O adopts the anti-cadmium iodide structure in the solid state. In this way, the Tl(I) centers are pyramidal...
3 KB (233 words) - 03:25, 20 July 2025
697×10−4 Cadmium iodate Cd(IO3)2 0.097 Cadmium iodide CdI2 78.7 84.7 87.9 92.1 100 111 125 Cadmium nitrate Cd(NO3)2 122 136 150 194 310 713 Cadmium oxalate...
84 KB (284 words) - 04:33, 30 June 2025
The anhydrous salts adopt the cadmium halide structures. The hexaaquo salt consists of separated [Co(H2O)6]2+ and iodide ions as verified crystallographically...
6 KB (388 words) - 19:36, 27 October 2022
as a result of the oxidation of the iodide ion to iodine. It has a trigonal crystal structure of the cadmium iodide type (polytype 2H) with the space group...
6 KB (392 words) - 02:37, 28 May 2025
example, magnesium hydroxide Mg(OH)2 (brucite) crystallizes with the cadmium iodide layer structure, with a kind of close-packing of magnesium and hydroxide...
41 KB (4,863 words) - 17:58, 31 July 2025
iodide oxides and release hydrogen gas. This process occurs faster in the presence of water. This compound has the same crystal structure as cadmium chloride...
4 KB (284 words) - 04:06, 28 May 2025
by thermal decomposition of chromium(III) iodide. Like many metal diiodides, CrI2 adopts the "cadmium iodide structure" motif, i.e., it features sheets...
3 KB (224 words) - 08:26, 20 June 2025