Calcium nitrate are inorganic compounds with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the...
12 KB (1,038 words) - 19:31, 1 April 2024
Calcium ammonium nitrate or CAN, also known as nitro-limestone or nitrochalk, is a widely used inorganic fertilizer, accounting for 4% of all nitrogen...
6 KB (443 words) - 14:45, 6 April 2024
rockets. Calcium nitrate, or lime saltpetre, was discovered on the walls of stables, from the urine of barnyard animals. Potassium nitrate was produced...
49 KB (4,959 words) - 12:21, 3 June 2024
UAN (redirect from Urea ammonium nitrate)
Urea–ammonium nitrate solutions should not be combined with calcium ammonium nitrate (CAN-17) or other solutions prepared from calcium nitrate. A thick, milky-white...
2 KB (212 words) - 14:02, 30 June 2023
calcium nitrate produces nitrogen fertilizer. The filtrate is composed mainly of phosphoric acid with some nitric acid and traces of calcium nitrate,...
3 KB (463 words) - 21:37, 11 January 2024
products, calcium carbonate and ammonium nitrate, may be separately purified or sold combined as calcium ammonium nitrate. Ammonium nitrate can also be...
29 KB (2,566 words) - 16:04, 31 May 2024
Fertilizer blends containing magnesium nitrate also have ammonium nitrate, calcium nitrate, potassium nitrate and micronutrients in most cases; these...
6 KB (363 words) - 19:53, 9 February 2024
Strontium nitrate is an inorganic compound composed of the elements strontium, nitrogen and oxygen with the formula Sr(NO3)2. This colorless solid is...
5 KB (307 words) - 00:31, 6 February 2024
or nitratine, the mineral form Norwegian saltpeter or calcium nitrate (Ca(NO3)2) Magnesium nitrate (Mg(NO3)2) Saltpetre Republic, a term used in Chilean...
598 bytes (98 words) - 08:37, 21 June 2022
calcium perchlorate (Ca(ClO4)2), calcium hexafluorophosphate (Ca(PF6)2), and calcium nitrate (Ca(NO3)2). Calcium nitrate is commonly used in aqueous batteries...
33 KB (3,890 words) - 05:22, 8 April 2024
Giovanni; Amico, Antonio (1985). "Transport properties of lithium nitrate and calcium nitrate binary solutions in molten acetamide". Journal of Chemical &...
8 KB (578 words) - 22:28, 17 December 2023
Nitric acid (redirect from Hydrogen nitrate)
around 1913. His method produced nitric acid from electrolysis of calcium nitrate converted by bacteria from nitrogenous matter in peat bogs. An earthenware...
46 KB (5,165 words) - 17:24, 27 May 2024
Fertilizer (redirect from Nitrate pollution)
it is solid and non-explosive, unlike ammonia and ammonium nitrate. Calcium ammonium nitrate (Ca(NO3)2 · NH4 · 10 H2O), reportedly holding a small share...
95 KB (10,208 words) - 20:51, 30 May 2024
such as barium, strontium, calcium, silver, or lead. e.g. Al2(SO4)3 + 3 Ba(NO3)2 → 2 Al(NO3)3 + 3 BaSO4. Aluminium nitrate is a strong oxidizing agent...
5 KB (290 words) - 22:27, 17 December 2023
applications of calcium nitrate at 200 ppm nitrogen, for example. Soil pH should be tested, and corrected if needed, because calcium deficiency is often...
11 KB (1,215 words) - 15:16, 26 April 2024
It is sometimes added to blends which contain calcium nitrate, magnesium nitrate and potassium nitrate to produce water-soluble formulas such as 15-5-15...
2 KB (179 words) - 01:23, 3 March 2024
Hydroxyapatite (redirect from Calcium hydroxyapatite)
anions. Preparation of these calcium-deficient phases can be prepared by precipitation from a mixture of calcium nitrate and diammonium phosphate with...
36 KB (3,775 words) - 20:18, 16 March 2024
acid as well as calcium nitrite. Also, it can be prepared as detailed below, forming a solution of sodium nitrite and calcium nitrate; cooling the solution...
8 KB (840 words) - 08:17, 8 November 2023
water) which will remove the buildup of calcium compound crystals, such as calcium carbonate or calcium nitrate. Excessive buildup can reduce the effectiveness...
10 KB (1,329 words) - 21:05, 23 May 2024
Nitrate is a polyatomic ion with the chemical formula NO− 3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers...
31 KB (3,501 words) - 02:12, 1 June 2024
with highly hygroscopic calcium nitrate), or if the powder was simply old (due to the mildly hygroscopic nature of potassium nitrate), in humid weather it...
95 KB (11,630 words) - 01:14, 5 June 2024
phosphate, 400 mg potassium chloride, 100 mg magnesium sulfate, and 100 mg calcium nitrate) Amino acids (300 mg glutamine; 200 mg arginine; 50 mg each asparagine...
3 KB (445 words) - 00:08, 29 November 2023
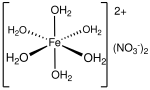
the compound is produced by the reaction of iron(II) chloride and calcium nitrate: FeCl2 + Ca(NO3)2 → Fe(NO3)2 + CaCl2 The hexahydrate melts at 60 °C...
8 KB (732 words) - 12:30, 3 April 2024
Niter (category Nitrate minerals)
structure resembles that of aragonite, with potassium replacing calcium and nitrate replacing carbonate. It occurs in the soils of arid regions and as...
13 KB (1,395 words) - 22:25, 2 June 2024
nitric acid, guanidine nitrate is produced industrially by the reaction of dicyandiamide (or calcium salt) and ammonium nitrate. It has been used as a...
5 KB (406 words) - 18:38, 17 April 2024
The function of this solution is to create a hydrophilic layer of calcium nitrate salt, Ca(NO 3) 2, and gum arabic on all non-image surfaces. The gum...
23 KB (2,893 words) - 01:32, 29 May 2024
acceleration today are calcium nitrate (Ca(NO3)2), calcium nitrite (Ca(NO2)2), calcium formate (Ca(HCOO)2) and aluminium compounds. Calcium chloride (CaCl2)...
4 KB (488 words) - 16:12, 4 April 2024
tetrahydrate can be prepared by reacting sodium pyrophosphate, Na4P2O7 with calcium nitrate, Ca(NO3)2, at carefully controlled pH and temperature: Na4P2O7(aq)+2...
8 KB (653 words) - 14:15, 19 March 2024
chemical reaction between the two substances, such as the reaction of calcium hydroxide with hydrochloric acid; even though one might say, informally...
50 KB (6,532 words) - 15:42, 7 June 2024
of osteoclast activity, thus lowering the amount of free calcium in the blood. Gallium nitrate is also used to synthesize other gallium compounds. Gallium...
12 KB (1,255 words) - 23:21, 23 August 2023