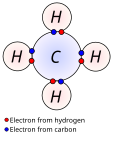
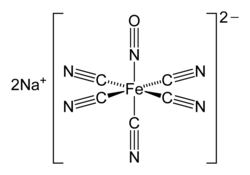
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...
14 KB (1,701 words) - 03:27, 4 October 2023
In chemistry, electron counting is a formalism for assigning a number of valence electrons to individual atoms in a molecule. It is used for classifying...
14 KB (1,629 words) - 15:35, 19 January 2025
complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin...
11 KB (1,364 words) - 02:22, 13 April 2025
VSEPR theory (redirect from Valence shell electron pair repulsion)
Some transition metal complexes with low d electron count have unusual geometries, which can be ascribed to d subshell bonding interaction. Gillespie found...
45 KB (4,059 words) - 10:26, 17 May 2025
the fact that the valence orbitals in the electron configuration of transition metals consist of five (n−1)d orbitals, one ns orbital, and three np orbitals...
17 KB (1,923 words) - 10:21, 9 April 2025
metals. The d electron count is an alternative tool for understanding the chemistry of a transition metal. The number of valence electrons of an element...
24 KB (2,333 words) - 15:26, 27 November 2024
elimination, the oxidation state of the metal decreases by two, while the d-electron count of the metal increases by two. This pathway is common for d8 metals...
13 KB (1,441 words) - 22:05, 12 November 2024
ground-state electron configurations of the elements were experimentally determined. Born–Oppenheimer approximation d electron count Electron configurations...
60 KB (6,206 words) - 11:54, 27 April 2025
have a distinct preference for the octahedral site depending on the d-electron count. If the A2+ ions have a strong preference for the octahedral site,...
16 KB (2,026 words) - 22:53, 14 May 2025
valence electrons. In certain transition metal complexes with a low d electron count, the p-orbitals are unoccupied and sdx hybridisation is used to model...
33 KB (3,204 words) - 08:17, 15 March 2025
atoms in organometallic compounds are frequently described by their d electron count and oxidation state. These concepts can be used to help predict their...
31 KB (3,163 words) - 08:57, 10 May 2025
reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy....
39 KB (3,992 words) - 01:59, 5 April 2025
oxidation states with low d electron counts. The high oxidation state stabilizes the highly reduced ligands. The low d electron count allow for many bonds...
7 KB (1,027 words) - 18:43, 11 October 2024
2). Using the neutral electron counting scheme, Cr has 6 d electrons and NO· has one electron for a total of 7. Two electrons are subtracted to take...
18 KB (2,128 words) - 16:41, 4 April 2025
complexes by Houser et al. and also Okuniewski et al. In systems with low d electron count, due to special electronic effects such as (second-order) Jahn–Teller...
57 KB (5,547 words) - 23:59, 15 February 2025
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to...
28 KB (2,765 words) - 11:55, 25 April 2025
covalent bonds, electrons shared between two atoms are counted toward the octet of both atoms. In carbon dioxide each oxygen shares four electrons with the central...
23 KB (2,876 words) - 21:11, 7 May 2025
In chemistry the polyhedral skeletal electron pair theory (PSEPT) provides electron counting rules useful for predicting the structures of clusters such...
20 KB (2,190 words) - 16:15, 15 October 2024
by a number of electrons that neutralize its charge. Electrons are bound to the nucleus to different degrees: the least bound electrons are responsible...
154 KB (15,743 words) - 00:06, 8 May 2025
d-electron count". Journal of Organometallic Chemistry. 394 (1–3): 777–794. doi:10.1016/0022-328X(90)87268-I. Atwood, J. L.; Hunter, W. E.; Hrncir, D...
12 KB (1,349 words) - 17:46, 30 September 2023
complexes of the type [MCl2(py)4]2+ reveal an anticorrelation with d-electron count. Low-valent metal complexes of pyridines are known, e.g. IrI(diene)(pyridine)Cl...
22 KB (2,001 words) - 11:46, 26 February 2025
Lewis structure (redirect from Electron Dot Structure)
adding or removing electrons to/from the appropriate atoms. A trick is to count up valence electrons, then count up the number of electrons needed to complete...
16 KB (2,159 words) - 05:20, 16 May 2025
attached via oxygen (tBu3SiO−)) ligands. The tantalum center has a d-electron count of 2 and an oxidation state of III. The complex is trigonal planar...
12 KB (1,317 words) - 16:15, 19 January 2025
complexes are known for metal centers with octahedral symmetry and d-electron counts beyond 5. Oxo compounds for the vanadium through iron triads (Groups...
20 KB (1,893 words) - 23:32, 14 January 2025
due to their highly filled d-orbitals, or high d-electron counts, to promote metal to ligand charge transfer from pi-electron accepting ligands. This interaction...
29 KB (3,115 words) - 19:54, 11 May 2025