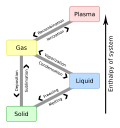
Deposition is the phase transition in which gas transforms into solid without passing through the liquid phase. Deposition is a thermodynamic process....
3 KB (416 words) - 15:35, 13 June 2025
and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another...
54 KB (6,649 words) - 18:05, 25 May 2025
two-step phase transition ― a solid-to-gas transition (sublimation in a more precise definition) followed by a gas-to-solid transition (deposition). (See...
23 KB (2,429 words) - 02:37, 7 April 2025
settling out of a solution Deposition (geology), material such as sediment being added to a landform Deposition (phase transition), the process by which a...
4 KB (546 words) - 19:42, 27 August 2024
dampness from coming into the line at any point along its length. Deposition (phase transition) List of desiccants Hygroscopy Mummy Reinicke, Kurt M.; Hueni...
8 KB (840 words) - 03:45, 15 March 2025
arrival rate of atomic species during deposition, this technique is especially useful to create a gradual transition between the substrate material and the...
2 KB (186 words) - 08:36, 22 December 2024
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas-phase chemical process; it is a subclass of chemical...
65 KB (7,440 words) - 04:51, 17 February 2025
State of matter (category Phases of matter)
phase transition. Water can be said to have several distinct solid states. The appearance of superconductivity is associated with a phase transition,...
36 KB (4,416 words) - 16:40, 4 June 2025
Condensation (category Phase transitions)
the atmosphere. When the transition happens from the gaseous phase into the solid phase directly, the change is called deposition. Condensation is usually...
9 KB (976 words) - 21:17, 20 May 2025
process in which the material transitions from a condensed phase to a vapor phase and then back to a thin film condensed phase. The most common PVD processes...
17 KB (1,868 words) - 09:39, 2 May 2025
(turning into a solid): Freezing, the solidification of a liquid Deposition (phase transition), solidification of a gas This disambiguation page lists articles...
432 bytes (87 words) - 22:02, 18 December 2018
Evaporation (category Phase transitions)
layer, where the phase is undetermined. Because this layer is only a few molecules thick, at a macroscopic scale a clear phase transition interface cannot...
15 KB (1,798 words) - 19:16, 2 March 2025
existence of a proton-ordered ferroelectric phase. However, they could not conclusively prove that a phase transition had taken place, and Onsager pointed out...
137 KB (15,379 words) - 17:52, 9 June 2025
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often...
42 KB (5,024 words) - 16:41, 21 May 2025
Epitaxy (redirect from Epitaxial deposition)
(prefix epi- means "on top of”) is a type of crystal growth or material deposition in which new crystalline layers are formed with one or more well-defined...
30 KB (3,623 words) - 17:56, 29 May 2025
Thin film (redirect from Thin-film deposition)
electroplating, and the deposition of silicon and enriched uranium by a chemical vapor deposition-like process after gas-phase processing. Deposition techniques fall...
76 KB (9,740 words) - 04:41, 2 June 2025
Boiling (category Phase transitions)
Boiling or ebullition is the rapid phase transition from liquid to gas or vapour; the reverse of boiling is condensation. Boiling occurs when a liquid...
18 KB (2,274 words) - 17:08, 9 May 2025
by vapor deposition, Perhaps there must be a phase transition before the entropy of the liquid decreases. In this scenario, the transition temperature...
73 KB (8,849 words) - 03:16, 9 June 2025
Freezing (category Phase transitions)
Freezing is a phase transition in which a liquid turns into a solid when its temperature is lowered below its freezing point. For most substances, the...
12 KB (1,345 words) - 06:18, 3 June 2025
Vaporization (category Phase transitions)
Vaporization (or vapo(u)risation) of an element or compound is a phase transition from the liquid phase to vapor. There are two types of vaporization: evaporation...
5 KB (606 words) - 09:19, 11 May 2025
advances in controlled synthesis of two-dimensional transition metal dichalcogenides via vapour deposition techniques". Chemical Society Reviews. 44 (9): 2744–2756...
96 KB (10,546 words) - 04:41, 9 June 2025
(album), by Canvas Solaris, 2004 Sublimation (phase transition), directly from the solid to the gas phase Sublimation (psychology), a mature type of defense...
650 bytes (114 words) - 15:41, 28 January 2025
the basin. Towards the end of this phase, the shoreline began to retreat and deposition continued. After the deposition of the pre-Turonian units, there...
29 KB (3,480 words) - 07:26, 11 May 2025
Crystallization (category Phase transitions)
routes including precipitation from solution, freezing of a liquid, or deposition from a gas. Attributes of the resulting crystal can depend largely on...
31 KB (3,943 words) - 21:10, 16 June 2025
Electron-beam physical vapor deposition, or EBPVD, is a form of physical vapor deposition in which a target anode is bombarded with an electron beam given...
13 KB (1,776 words) - 08:55, 13 April 2025
Liquidus and solidus (redirect from Primary crystalline phase)
"Measurements of the liquidus surface and solidus transitions of the NaCl–UCl3 and NaCl–UCl3–CeCl3 phase diagrams". Journal of Nuclear Materials. 466. Elsevier...
10 KB (1,149 words) - 04:14, 19 November 2024
Melting (category Phase transitions)
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy...
10 KB (1,235 words) - 02:03, 25 March 2025
Cadmium arsenide (section Phase transition)
716 °C and changes phase at 615 °C/ Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable),...
15 KB (1,450 words) - 04:36, 2 April 2025
Triple point (category Phase transitions)
in that it has no sublimation/deposition curve and therefore no triple points where its solid phase meets its gas phase. Instead, it has a vapor-liquid-superfluid...
17 KB (1,349 words) - 01:44, 31 May 2025
reduction of catalyst surface area. Fouling: the deposition of materials from the fluid phase onto the solid phase catalyst and/or support surfaces. This results...
30 KB (3,246 words) - 03:32, 23 May 2025