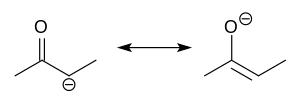
enol ether is an alkene with an alkoxy substituent. The general structure is R2C=CR-OR where R = H, alkyl or aryl. A common subfamily of enol ethers are...
7 KB (807 words) - 11:17, 14 April 2025
In organosilicon chemistry, silyl enol ethers are a class of organic compounds that share the common functional group R3Si−O−CR=CR2, composed of an enolate...
13 KB (1,369 words) - 22:56, 11 March 2025
electrophiles at oxygen. Silylation gives silyl enol ether. Acylation gives esters such as vinyl acetate. In general, enols are less stable than their keto equivalents...
14 KB (1,159 words) - 01:14, 13 March 2025
Ge, Sn, Pb). Such compounds are considered ethers as well. Examples of such ethers are silyl enol ethers R3Si−O−CR=CR2 (containing the Si−O−C linkage)...
19 KB (1,841 words) - 05:47, 17 June 2025
Thebaine (redirect from Codeine methyl enol ether)
Thebaine (paramorphine), also known as codeine methyl enol ether, is an opiate alkaloid, its name coming from the Greek Θῆβαι, Thēbai (Thebes), an ancient...
11 KB (730 words) - 14:16, 19 February 2025
Ethyl vinyl ether is an organic compound with the chemical formula CH3CH2OCH=CH2. It is the simplest enol ether that is liquid at room temperature. It...
3 KB (274 words) - 16:40, 22 February 2025
oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product...
28 KB (3,396 words) - 17:19, 26 September 2024
Progestogen ester (redirect from Progesterone ether)
3-cyclopentyl enol ethers of 17α-hydroxyprogesterone and 17α-hydroxyprogesterone acetate, respectively, while progesterone 3-acetyl enol ether (never marketed)...
22 KB (2,387 words) - 00:35, 9 December 2023
Methyl vinyl ether is an organic compound with the chemical formula CH3OCH=CH2. A colorless gas, it is the simplest enol ether. It is used as a synthetic...
6 KB (473 words) - 16:41, 22 February 2025
addition is an organic reaction and a type of aldol reaction between a silyl enol ether (R2C=CR−O−Si(CH3)3) and an aldehyde (R−CH=O) or formate (R−O−CH=O). The...
9 KB (958 words) - 06:34, 11 February 2024
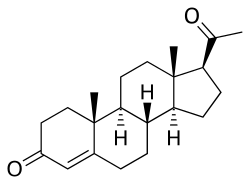
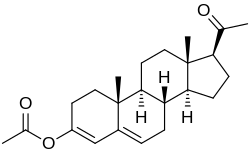
Progesterone 3-acetyl enol ether, also known as progesterone acetate, as well as 3-acetoxypregna-3,5-dien-20-one, is a progestin which was never marketed...
7 KB (548 words) - 08:17, 12 February 2025
Self-condensation (section Silyl enol ether formation)
occur. One way to get around this is to turn the aldehyde into a silyl enol ether using trimethylsilyl chloride and a base, such as triethylamine, and then...
4 KB (488 words) - 13:28, 23 December 2024
"Regio- AND Stereoselective Intramolecular Hydrosilylation of a-Hydroxy Enol Ethers: 2,3-syn-2-Methoxymethoxy-1,3-nonanediol". Organic Syntheses. 73: 94...
4 KB (408 words) - 07:36, 16 May 2025
Silyl triflate is more reactive and also converts ketones to silyl enol ethers. Silyl triflates are water sensitive and must be run under inert atmosphere...
10 KB (1,269 words) - 23:02, 25 May 2025
is transformed into an enol ether by the Tebbe reagent (Cp2Ti=CH2), and then the glycosyl acceptor is tethered to the enol ether under acid-catalysed conditions...
7 KB (907 words) - 17:26, 22 October 2017
antioxidant activity comes from the enol ether double bond being targeted by a variety of reactive oxygen species. Synthetic ether lipid analogs have cytostatic...
13 KB (1,497 words) - 04:54, 14 June 2025
intermediate between that of an enol ether and an aromatic ring. It is dissimilar vs ethers such as tetrahydrofuran. Like enol ethers, 2,5-disubstituted furans...
19 KB (1,513 words) - 10:14, 21 May 2025
1990). "Triethylborane induced perfluoroalkylation of silyl enol ethers or germyl enol ethers with perfluoroalkyl iodides". Tetrahedron Letters. 31 (44):...
45 KB (4,266 words) - 16:35, 14 June 2025
instead of an alkene yields an enol, which tautomerizes into a ketone. Using an alcohol instead of water yields an ether (see also Hofmann-Sand reaction)...
10 KB (1,156 words) - 03:06, 17 October 2024
by electron donating groups proceed rapidly and easily. For example, enol ethers like trimethylsilyloxy-substituted olefins are often used because of...
29 KB (2,981 words) - 20:48, 22 May 2025
Ketone (section Keto-enol tautomerization)
that have at least one alpha-hydrogen, undergo keto-enol tautomerization; the tautomer is an enol. Tautomerization is catalyzed by both acids and bases...
24 KB (2,895 words) - 18:04, 2 June 2025
Aldol reactions (section Enol mechanism)
Carbonyl compounds, such as aldehydes and ketones, can be converted to enols or enol ethers. These species, being nucleophilic at the α-carbon, can attack especially...
9 KB (957 words) - 15:23, 19 January 2025
Rifampicin (category Enol ethers)
Rifampicin, also known as rifampin, is an ansamycin antibiotic used to treat several types of bacterial infections, including tuberculosis (TB), Mycobacterium...
48 KB (4,812 words) - 22:05, 29 May 2025
Methyltestosterone 3-hexyl ether (brand names Androgénol, Enoltestovis, Enoltestovister), or 17α-methyltestosterone 3-hexyl enol ether, also known as 17α-methylandrost-3...
3 KB (84 words) - 06:14, 14 January 2025
Vinyl ether may refer to: Any enol ether Divinyl ether, a volatile chemical compound once used as an anestethic This disambiguation page lists articles...
146 bytes (51 words) - 20:29, 13 April 2019
Aldol reaction (section On the enol)
uses other, similar functional groups as ersatz enols. In the Mukaiyama aldol reaction, silyl enol ethers add to carbonyls in the presence of a Lewis acid...
40 KB (4,256 words) - 12:17, 6 June 2025
Quingestrone (redirect from Progesterone cyclopentyl enol ether)
Quingestrone, also known as progesterone 3-cyclopentyl enol ether (PCPE) and sold under the brand name Enol-Luteovis, is a progestin medication which was previously...
21 KB (1,940 words) - 07:41, 29 April 2025
compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield...
13 KB (1,392 words) - 19:41, 10 February 2025
Azoxystrobin (category Enol ethers)
Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using...
28 KB (2,784 words) - 15:40, 29 May 2025
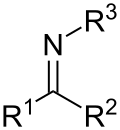
intermediates in the synthesis of heterocycles. Aromatic imines react with an enol ether to a quinoline in the Povarov reaction. Imines react, thermally, with...
24 KB (2,710 words) - 15:58, 26 May 2025