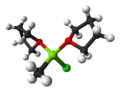
In organometallic chemistry, metal–halogen exchange is a fundamental reaction that converts an organic halide into an organometallic product. The reaction...
10 KB (1,200 words) - 17:34, 25 September 2023
Organosodium chemistry (section Metal-halogen exchange)
+ NaH → CH3SOCH− 2Na+ + H2 Trityl sodium can be prepared by sodium-halogen exchange: Ph3CCl + 2 Na → Ph3C− Na+ + NaCl Sodium also reacts with polycyclic...
11 KB (1,261 words) - 00:12, 27 August 2023
"Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions". Organic Syntheses. 89: 460. doi:10.15227/orgsyn.089...
24 KB (2,721 words) - 12:37, 30 April 2025
limited due to competing side reactions such as radical reactions or metal–halogen exchange. Most organolithium reagents used in alkylations are more stabilized...
55 KB (5,955 words) - 23:00, 13 March 2025
compounds through metal-halogen exchange.: 106 Unlike the organolithium compounds, the organometallic compounds of the heavier alkali metals are predominantly...
225 KB (24,165 words) - 17:24, 9 May 2025
replacing the proton. 2-Lithiothiazoles are also generated by metal-halogen exchange from 2-bromothiazole. Electrophilic aromatic substitution at C5...
12 KB (1,151 words) - 18:44, 12 March 2025
aside from the use of metal acetylides as nucleophiles, such a process rarely works well in practice due to metal–halogen exchange and/or the formation...
11 KB (1,505 words) - 20:21, 12 July 2024
Phenylsodium (section Metal-halogen exchange)
the Alkali Metals: Useful Synthetic Reagents as Strong Bases and Potent Nucleophiles. 1. Conversion of Organic Halides to Organoalkali-Metal Compounds"...
9 KB (981 words) - 14:39, 12 February 2025
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides...
13 KB (1,440 words) - 21:35, 4 September 2024
with metals, generally lithium or magnesium, to give organometallic derivatives that function as sources of aryl anions. By the metal–halogen exchange reaction...
17 KB (1,924 words) - 17:37, 11 March 2025
undergo metal-halogen exchange and then eliminate a lithium salt: R2CBr2 + BuLi → R2CLi(Br) + BuBr R2CLi(Br) → R2C + LiBr Zinc metal abstracts halogens similarly...
22 KB (2,421 words) - 15:05, 18 March 2025
synthesised via a much more controllable (and cheaper) route, via metal-halogen exchange and then metathesis: Br(CH2)3Br + 2 tBuLi → Li(CH2)3Li + 2 tBuBr...
4 KB (350 words) - 12:36, 28 September 2023
representative of reductive coupling. The reaction proceeds by an initial metal–halogen exchange, which is described with the following idealized stoichiometry:...
5 KB (534 words) - 11:04, 29 April 2025
A metal (from Ancient Greek μέταλλον (métallon) 'mine, quarry, metal') is a material that, when polished or fractured, shows a lustrous appearance, and...
97 KB (11,444 words) - 12:02, 9 May 2025
"Preparation of t-Butyl-3-Bromo-5-Formylbenzoate Through Selective Metal-Halogen Exchange Reactions". Organic Syntheses. 89: 460. doi:10.15227/orgsyn.089...
17 KB (2,004 words) - 11:39, 24 January 2025
N-Butyllithium (section Halogen–lithium exchange)
products of the corresponding halogen–lithium exchanges (C4H9Br or C4H9Cl). Other metals and metalloids which undergo such exchange reactions are organic compounds...
18 KB (1,858 words) - 14:40, 25 April 2025
with a reducing metal, such as sodium or magnesium, or with an organolithium reagent. Either approach results in metal-halogen exchange to convert the...
4 KB (392 words) - 08:49, 18 February 2025
Dehalogenation (category Halogens)
interest in organic synthesis, electropositive metals react with many organic halides in a metal-halogen exchange: RX + 2 M → RM + MX The resulting organometallic...
9 KB (886 words) - 09:31, 7 August 2024
perform nucleophilic addition, substitution, transmetalation, and metal-halogen exchange reactions. The first crystal structure of Grignard reagents was...
61 KB (5,969 words) - 01:07, 20 January 2025
post-transition metals, poor metals, other metals, p-block metals, basic metals, and chemically weak metals. The most common name, post-transition metals, is generally...
125 KB (15,308 words) - 22:06, 30 April 2025
chemical terms, all of the alkaline earth metals react with the halogens to form the alkaline earth metal halides, all of which are ionic crystalline...
72 KB (7,359 words) - 02:51, 7 April 2025
and aryls from metallic lithium and halogenated hydrocarbons via metal–halogen exchange. This convenient synthesis spurred numerous studies of RLi reagents...
23 KB (2,670 words) - 09:37, 15 March 2025
coordinate ligands other than halogens to the metal: 2 U + 3 (C5H5)2Hg + HgCl2 → 2 (C5H5)3UCl + 4 Hg Alkaline earth metal complexes have been synthesized...
13 KB (1,548 words) - 14:33, 18 December 2021
Transition metals in the periodic table In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic...
40 KB (4,500 words) - 18:36, 26 February 2025
less reactive halogen cannot replace the more reactive halogen: I 2 + 2 KBr ⟶ {\displaystyle {\ce {I2 + 2KBr ->}}} no reaction Metals react with acids...
11 KB (1,329 words) - 22:42, 23 April 2025
2 Li → Ph−Li + LiX Phenyllithium can also be synthesized with a metal-halogen exchange reaction: n-BuLi + Ph−X → n-BuX + Ph−Li The predominant method of...
7 KB (663 words) - 16:25, 13 February 2025
Bromine (category Halogens)
tetrabromide at 370 °C to form niobium(V) bromide. Another method is halogen exchange in the presence of excess "halogenating reagent", for example: FeCl3...
67 KB (7,754 words) - 17:16, 8 May 2025
replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the laboratory, metalation is commonly...
11 KB (722 words) - 10:04, 16 October 2024
bonds are cleaved by halogens. Depending on the electron-counting scheme used, this can be regarded as an oxidation of the metal atoms: Mn2(CO)10 + Cl2...
71 KB (7,973 words) - 23:53, 15 May 2025