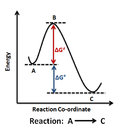
mechanism and choice of rate-determining step is clear. The correct rate-determining step can be identified by predicting the rate law for each possible...
12 KB (1,736 words) - 20:02, 2 September 2024
Kinetic isotope effect (section Evaluation of rate constant ratios from intermolecular competition reactions)
bond cleavage occurs during the rate-determining step or at a product-determining step ensuing the rate-determining step. The absence of a KIE, at least...
100 KB (14,024 words) - 20:28, 24 May 2025
mechanism, which means both the reacting species are involved in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic...
21 KB (2,560 words) - 11:49, 26 June 2025
{M}}]} , which is second order. That is, the rate-determining step is the first, bimolecular activation step. At higher pressures, however, k − 1 [ M ]...
11 KB (1,677 words) - 08:20, 29 May 2025
Enzyme kinetics (redirect from Rate of enzyme mediated reactions)
of steps, there is typically one rate-determining step that determines the overall kinetics. This rate-determining step may be a chemical reaction or a...
72 KB (9,551 words) - 07:46, 27 March 2025
may change the rate-determining step (rds) in the mechanism of the reaction. A certain electronic effect may accelerate a certain step so that it is no...
34 KB (4,055 words) - 16:23, 22 April 2025
SN1 reaction (section Rate law)
"nucleophilic substitution", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation is often shown as having first-order dependence...
15 KB (1,978 words) - 11:47, 26 June 2025
coefficient. It is rare that the first committed step is in fact the rate-determining step. Metabolic pathways require tight regulation, so that the proper compounds...
7 KB (675 words) - 22:45, 17 February 2025
does not appear in the rate equation because it reacts in the third step, which is a rapid step after the rate-determining step, so that it does not affect...
26 KB (3,726 words) - 15:07, 12 June 2025
When determining the overall rate law for a reaction, the slowest step is the step that determines the reaction rate. Because the first step (in the...
14 KB (1,617 words) - 15:36, 24 June 2025
iodate and bisulfite: IO−3 + 3 HSO−3 → I− + 3 HSO−4 This first step is the rate determining step. Next, the iodate in excess will oxidize the iodide generated...
9 KB (992 words) - 07:33, 25 May 2025
evidence points to reaction 2 as being slow, rate-determining step. This is not unexpected, since that step breaks the nitrogen triple bond, the strongest...
72 KB (8,393 words) - 18:40, 17 June 2025
reacting system increases in the rate-determining step. Dissociative pathways are characterized by a rate determining step that involves release of a ligand...
12 KB (1,456 words) - 18:28, 20 March 2025
will determine the rate of the reaction. This step of the reaction whose rate determines the overall rate of reaction is known as rate determining step or...
29 KB (3,603 words) - 15:06, 13 February 2024
Another type of mixed-order rate law has a denominator of two or more terms, often because the identity of the rate-determining step depends on the values of...
44 KB (7,578 words) - 06:10, 25 May 2025
substituents and applying Hammond's postulate it was concluded that the rate-determining step involves formation of a transition state that should resemble the...
20 KB (2,564 words) - 14:11, 26 May 2025
that the first equilibrium is not the rate-determining step. Further experiments showed a larger relative rate in a deuterated solvent system compared...
8 KB (950 words) - 02:17, 14 May 2025
E1cB-elimination reaction (section Rate law)
lose two substituents. Unimolecular refers to the fact that the rate-determining step of this reaction only involves one molecular entity. Finally, conjugate...
18 KB (2,154 words) - 09:15, 26 June 2025
use a pre-formed hydrazone as substrate (see modifications). The rate determining step of the reaction is de-protonation of the hydrazone by an alkoxide...
41 KB (4,214 words) - 14:06, 20 May 2025
reactants. The product determining step is not rate limiting if the rate limiting step of each mechanism is the same. Rate-determining step Louden, Marc G. Organic...
586 bytes (68 words) - 16:07, 9 October 2023
concentrated sulfuric acid (H2SO4). The second major step, which is also the rate-determining step, is the annulation of the molecule. Immediately following...
11 KB (1,216 words) - 10:09, 25 June 2025
Stille reaction (section Reductive elimination step)
transmetalation step is normally the rate-determining step. Larry Overman and coworkers make use of the Stille-carbonylative cross-coupling in their 20-step enantioselective...
47 KB (5,493 words) - 02:16, 16 May 2025
rate determining step. Hydride ion elimination In addition to the mechanism shown above, other pathways have been proposed for the elimination step....
8 KB (1,062 words) - 00:03, 14 December 2024
bimolecular (i.e. there are two molecular species involved in the rate-determining step). The reaction does not have any intermediate steps, only a transition...
2 KB (211 words) - 11:56, 24 June 2025
always the rate-determining step. Hence, the activation energy and therefore rate of the reaction will depend only upon the dissociation step. First, consider...
31 KB (3,787 words) - 16:18, 25 May 2025
reacting system increases in the rate-determining step. Dissociative pathways are characterized by a rate determining step that involves release of a ligand...
6 KB (898 words) - 10:17, 26 June 2025
increasing electronegativity the reaction rate for nucleophilic attack increases. This is because the rate-determining step for an SNAr reaction is attack of...
11 KB (1,303 words) - 11:44, 26 June 2025
with the nucleophile since the nucleophile is not involved in the rate determining step. There are many reactions in organic chemistry involving this type...
13 KB (1,536 words) - 08:50, 10 May 2025
reaction and is calculated by Computational chemistry to be the rate-determining step in the oxidation. The twist mechanism also explains why oxidation...
13 KB (1,246 words) - 05:50, 7 June 2025
rate is influenced only by the concentration of the alkyl halide because carbocation formation is the slowest step, as known as the rate-determining step...
14 KB (1,853 words) - 12:34, 23 June 2025