The complex was first reported by J. W. DiLuzio and Lauri Vaska in 1961. Vaska's complex can undergo oxidative addition and is notable for its ability...
9 KB (812 words) - 19:34, 14 March 2022
chemistry of dioxygen. Such complexes can be generated by treating low-valent metal complexes with oxygen. For example, Vaska's complex reversibly binds O2 (Ph...
10 KB (1,086 words) - 09:15, 26 February 2025
became known as Vaska's complex, trans-IrCl(CO)[P(C6H5)3]2 Working with a series of coworkers, he demonstrated that this iridium(I) complex undergoes a variety...
6 KB (535 words) - 13:56, 4 February 2025
18-electron rule (redirect from 18-electron complex)
The most famous example is Vaska's complex (IrCl(CO)(PPh3)2), [PtCl4]2−, and Zeise's salt [PtCl3(η2-C2H4)]−. In such complexes, the dz2 orbital is doubly...
17 KB (1,923 words) - 10:21, 9 April 2025
Transition metal hydride (redirect from Metal hydride complex)
the addition of HCl to Vaska's complex: IrICl(CO)(PPh3)2 + HCl → HIrIIICl2(CO)(PPh3)2 Some metal hydrides form when a metal complex is treated with hydrogen...
23 KB (2,641 words) - 01:22, 5 October 2024
organometallic chemistry it is commonly used for the synthesis of Vaska's complex and related compounds such as carbonylchlorohydridotris(triphenylphosphine)ruthenium...
6 KB (417 words) - 13:09, 13 April 2025
An unusual cationic complex features three 16e Ru centers: 3 Cp*Ru(MeCN)3+ + C60 → {[(Cp*Ru(MeCN)2]3C60}3+ + 3 MeCN Vaska's complex forms a 1:1 adduct...
9 KB (934 words) - 13:15, 18 October 2024
the dioxygen derivative which forms reversibly upon oxygenation of Vaska's complex. The η-notation is encountered in many coordination compounds: Side-on...
13 KB (1,321 words) - 20:45, 16 January 2024
Square planar molecular geometry (redirect from Square-planar complex)
Wilkinson's catalyst and Crabtree's catalyst. Other examples include Vaska's complex and Zeise's salt. Certain ligands (such as porphyrins) stabilize this...
5 KB (451 words) - 18:58, 10 December 2024
Organoiridium chemistry (redirect from Organoiridium complex)
is Vaska's complex, bis(triphenylphosphine)iridium carbonyl chloride. Although iridium(I) complexes are often useful homogeneous catalysts, Vaska' complex...
11 KB (1,057 words) - 21:57, 27 March 2025
commonly found in the chemistry of 16e square planar metal complexes, e.g. Vaska's complex and tetrachloroplatinate. The rate law is governed by the Eigen–Wilkins...
12 KB (1,456 words) - 18:28, 20 March 2025
Triphenylphosphine (section Transition metal complexes)
chlorodiphenylphosphine (PPh2Cl). Tris(o-tolyl)phosphine Decyl(triphenyl)phosphonium Vaska's complex Wilkinson's catalyst Bis(triphenylphosphine)nickel(II) dichloride...
18 KB (1,624 words) - 21:47, 17 April 2025
the formation of an M–(C–H) agostic complex. A representative example is the reaction of hydrogen with Vaska's complex, trans-IrCl(CO)[P(C6H5)3]2. In this...
10 KB (1,210 words) - 14:57, 11 January 2025
colored blood-red) Tegillarca granosa - "blood clam" Vaska's complex – iridium organometallic complex notable for its ability to bind to O2 reversibly from...
98 KB (11,470 words) - 20:18, 1 May 2025
is a square planar Rh(I) complex of historical significance used to catalyze the hydrogenation of alkenes. Vaska's complex, trans-IrCl(CO)(PPh3)2, is...
14 KB (1,418 words) - 17:55, 22 June 2024
commonly found in the chemistry of 16e square planar metal complexes, e.g. Vaska's complex and tetrachloroplatinate. These compounds (MX4) bind the incoming...
10 KB (1,140 words) - 07:00, 8 March 2022
Bis(triphenylphosphine)rhodium carbonyl chloride (category Carbonyl complexes)
complex with the formula [RhCl(CO)(PPh3)2]. This complex of rhodium(I) is a bright yellow, air-stable solid. It is the Rh analogue of Vaska's complex...
4 KB (276 words) - 21:18, 8 January 2025
surgery Erythromer Induced blood stem cells Respirocyte Theatrical blood Vaska's complex: carries oxygen and hydrogen Cohn, Claudia S.; Cushing, Melissa M....
25 KB (2,747 words) - 02:11, 23 April 2025
Buckminsterfullerene (section Metal complexes)
precursors, such as Vaska's complex, for adducts with C60: trans-Ir(CO)Cl(PPh3)2 + C60 → Ir(CO)Cl(η2-C60)(PPh3)2 One such iridium complex,...
47 KB (4,568 words) - 14:57, 17 May 2025
to give transition metal acyl complexes. Illustrative is the oxidative addition of acetyl chloride to Vaska's complex, converting square planar Ir(I)...
20 KB (2,359 words) - 12:20, 4 February 2025
are prepared by oxidative addition: An example is the reaction of a Vaska's complex with methyl iodide. Some metal alkyls feature agostic interactions...
13 KB (1,037 words) - 13:59, 6 April 2024
carbonyls are prepared via decarbonylation reactions. The CO ligand in Vaska's complex arises by the decarbonylation of dimethylformamide: IrCl3(H2O)3 + 3...
8 KB (910 words) - 14:20, 13 August 2024
chloride is used in the for the preparation of other iridium complexes such as Vaska's complex, trans-[IrCl(CO)(PPh3)2]. With the presence of the chloride...
10 KB (784 words) - 23:40, 10 March 2025
iron pentacarbonyl, Zeise's salt, Vaska's complex, Wilkinson's catalyst. d9 Stable complexes with this electron count are more common for first...
14 KB (1,701 words) - 03:27, 4 October 2023
Dicarbonyltris(triphenylphosphine)ruthenium(0) (redirect from Roper's complex)
carrying only two CO ligands, the complex is highly nucleophilic. Many of its reactions parallel those for Vaska's complex. The derivative Ru(CO)2H2(PPh3)2...
6 KB (562 words) - 14:29, 3 July 2024
by X-ray crystallography. The dinitrogen complex trans-[IrCl(N2)(PPh3)2] is made by treating Vaska's complex with aromatic acyl azides. It has a planar...
18 KB (2,089 words) - 07:15, 5 December 2024
addition of acetyl chloride to Vaska's complex, converting square planar Ir(I) to octahedral Ir(III): Some acyl complexes can be produced from aldehydes...
6 KB (599 words) - 19:59, 23 February 2025
Rhodium(III) chloride (section Coordination complexes)
trans-RhCl(CO)(PPh3)2, stoichiometrically analogous to but less nucleophilic than Vaska's complex. trans-RhCl(CO)(PPh3)2 reacts with a mixture of NaBH4 and PPh3 to give...
19 KB (1,917 words) - 19:42, 27 January 2025
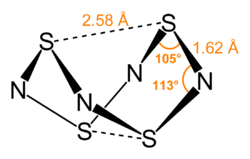
complex with S4N4 at sulfur. This compound, upon standing, isomerizes to additionally bond through a nitrogen atom. S4N4 oxidatively adds to Vaska's complex...
17 KB (1,632 words) - 03:28, 24 November 2024