Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH) 2, consisting of divalent cobalt cations Co2+ and hydroxide...
10 KB (860 words) - 15:17, 25 April 2025
Cobalt(III) hydroxide or cobaltic hydroxide is a chemical compound with formula Co(OH) 3 or H 3CoO 3. It is an ionic compound, with trivalent cobalt cations...
4 KB (255 words) - 22:03, 7 November 2023
III) oxide decomposes to cobalt(II) oxide at 950 °C: 2 Co3O4 → 6 CoO + O2 It may also be prepared by precipitating the hydroxide, followed by thermal...
5 KB (420 words) - 11:17, 5 March 2024
mutually trans aquo ligands. Cobalt chloride can be prepared in aqueous solution from cobalt(II) hydroxide or cobalt(II) carbonate and hydrochloric acid:...
21 KB (2,143 words) - 16:09, 26 May 2025
the cobalt recovery circuit and is purified by the removal of iron, copper, nickel, and zinc prior to the precipitation of cobalt as its hydroxide. This...
8 KB (1,079 words) - 01:11, 27 January 2025
Cobalt compounds are chemical compounds formed by cobalt with other elements. Many halides of cobalt(II) are known: cobalt(II) fluoride (CoF2) which is...
16 KB (1,842 words) - 04:49, 4 April 2025
Copper(II) hydroxide is the hydroxide of copper with the chemical formula of Cu(OH)2. It is a pale greenish blue or bluish green solid. Some forms of copper(II)...
14 KB (1,394 words) - 17:22, 18 January 2025
hydroxide Beryllium hydroxide Cobalt(II) hydroxide Copper(II) hydroxide Curium hydroxide Gold(III) hydroxide Iron(II) hydroxide Mercury(II) hydroxide...
2 KB (246 words) - 00:21, 12 January 2025
two cobalt(II) carbonate-hydroxides are known: Co2(CO3)(OH)2 and Co6(CO3)2(OH)8·H2O. The moderately rare spherocobaltite is a natural form of cobalt carbonate...
8 KB (573 words) - 21:25, 25 April 2025
chloride Calcium hydride Calcium oxide Calcium sulfate (Drierite) Cobalt(II) chloride Copper(II) sulfate Lithium chloride Lithium bromide Magnesium chloride...
1 KB (91 words) - 16:48, 2 October 2024
the monoclinic. It can be prepared by the reaction of cobalt(II,III) oxide (or cobalt(II) hydroxide) and germanium dioxide at high temperatures. The chemical...
3 KB (266 words) - 00:49, 27 May 2025
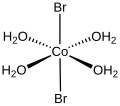
the formula trans-[CoBr2(H2O)4]. Cobalt(II) bromide can be prepared as a hydrate by the reaction of cobalt hydroxide with hydrobromic acid: Co(OH)2 +...
6 KB (351 words) - 05:19, 13 January 2025
fluoride – CoF2 Cobalt(II) hydroxide – Co(OH)2 Cobalt(II) iodide – CoI2 Cobalt(II) nitrate – Co(NO3)2 Cobalt(II) oxide – CoO Cobalt(II) perchlorate – Co(ClO4)2...
121 KB (8,924 words) - 03:42, 25 May 2025
Like many other transition metal acetates, cobalt(II) acetate forms by the reaction of cobalt oxide or hydroxide and acetic acid: CoO + 2 CH3CO2H + 3 H2O...
6 KB (434 words) - 10:11, 26 August 2024
Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is a cobalt(II) salt. The most common form is the hexahydrate Co(NO3)2·6H2O...
8 KB (588 words) - 08:29, 20 June 2025
Cobalt oxide/graphene composite are synthesized by first forming cobalt(II) hydroxide Co(OH) 2 on the graphene sheet from a cobalt(II) salt and...
9 KB (1,035 words) - 18:38, 23 September 2023
Since cobalt(II) has an odd number of electrons, its salts are paramagnetic. It forms by the reaction of metallic cobalt, its oxide, hydroxide, or carbonate...
10 KB (741 words) - 20:16, 10 September 2024
water and oxygen to brown cobalt(III) hydroxide (Co(OH)3). At temperatures of 600–700 °C, CoO oxidizes to the blue cobalt(II,III) oxide (Co3O4), which...
122 KB (12,558 words) - 04:06, 26 May 2025
with hard metal ions. Cobalt(II) squarate hydrate Co(C4O4)·2H2O (yellow, cubic) can be prepared by autoclaving cobalt(II) hydroxide and squaric acid in...
12 KB (1,207 words) - 16:33, 2 June 2025
CoCl2•6H2O or cobalt chloride hexahydrate is heated at 60 °C for 24 hours in a chemical bath deposition to form a brucite type cobalt(II) hydroxide layer with...
2 KB (257 words) - 07:26, 14 October 2023
cobalt(III) hydroxide 1307–86–4 CoS cobalt(II) sulfide 1317–42–6 CoS2 cobalt(IV) sulfide 12013–10–4 CoSe cobalt(II) selenide 1307–99–9 CoTe cobalt(II)...
139 KB (120 words) - 17:15, 19 May 2025
and bornhardtite (Co2+Co3+2Se4). Cobalt(II) selenide can be prepared by placing cobalt selenite in 10% sodium hydroxide solution, stirring it, and adding...
7 KB (654 words) - 23:41, 26 May 2025
15520-84-0 CoO cobalt(II) oxide 1307-96-6 Co(OH)2 cobalt(II) hydroxide 21041-93-0 Co(OH)3 cobalt(III) hydroxide 1307-86-4 CoS cobalt(II) sulfide 1317-42-6...
183 KB (107 words) - 18:05, 12 June 2025
formula [CuCl2+x]x−. When copper(II) chloride solutions are treated with a base, a precipitation of copper(II) hydroxide occurs: CuCl2 + 2 NaOH → Cu(OH)2...
30 KB (2,743 words) - 22:35, 23 May 2025
cobalt atoms have a formal charge of +3. The compound has been reported to exist in the gas phase at high temperatures, in equilibrium with cobalt(II)...
8 KB (911 words) - 08:51, 17 March 2024
Amphoterism (section Hydroxides)
zirconium, chromium, iron, cobalt, copper, silver, gold, germanium, antimony, bismuth, beryllium, and tellurium. Aluminium hydroxide is also amphoteric: Al...
9 KB (1,368 words) - 08:10, 2 June 2025
nickel(II) chloride with fluorine at 350 °C: NiCl2 + F2 → NiF2 + Cl2 The corresponding reaction of cobalt(II) chloride results in oxidation of the cobalt, whereas...
7 KB (466 words) - 01:24, 24 May 2025
Group 9 element (redirect from Cobalt family)
wear-resistant and high-strength alloys. The compounds cobalt silicate and cobalt(II) aluminate (CoAl2O4, cobalt blue) give a distinctive deep blue color to glass...
21 KB (2,226 words) - 22:06, 24 May 2025
calcination of hydrated cobalt oxalate β-CoC 2O 4·2H 2O, in the form of rod-like crystals about 8 μm long and 0.4 μm wide, with lithium hydroxide LiOH, up to 750–900...
12 KB (1,263 words) - 18:29, 3 June 2025
Sheet" (PDF) – via Connecticut College. Sigma-Aldrich Co., Nickel(II) carbonate hydroxide tetrahydrate. Retrieved on 2014-05-06. Keith Lascelles, Lindsay...
6 KB (480 words) - 19:34, 21 January 2025