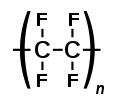
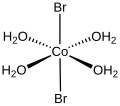
Cobalt(III) fluoride is the inorganic compound with the formula CoF3. Hydrates are also known. The anhydrous compound is a hygroscopic brown solid. It...
7 KB (610 words) - 13:30, 4 February 2025
produced in the reaction of cobalt (III) fluoride with water. The tetrahydrate cobalt(II) fluoride is formed by dissolving cobalt(II) in hydrofluoric acid...
6 KB (510 words) - 13:30, 4 February 2025
Cobalt(III) nitrate can be intercalated in graphite, in the ratio of 1 molecule for each 12 carbon atoms. Cobalt(III) fluoride Cobalt(III) chloride Cobalt(III)...
3 KB (321 words) - 17:07, 9 February 2025
their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride. The Manhattan Project required the production and handling of uranium...
6 KB (793 words) - 15:10, 23 May 2024
rather than the mixture of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. Terbium(IV) fluoride can be produced by the reaction...
6 KB (606 words) - 16:44, 17 May 2024
Iron(III) fluoride, also known as ferric fluoride, are inorganic compounds with the formula FeF3(H2O)x where x = 0 or 3. They are mainly of interest by...
10 KB (896 words) - 13:30, 4 February 2025
Cobalt fluoride may refer to: Cobalt(II) fluoride (cobalt difluoride), CoF2, red color Cobalt(III) fluoride (cobalt trifluoride), CoF3, brown color This...
502 bytes (55 words) - 09:20, 19 February 2017
of cobalt in the +3 oxidation state exist, such as cobalt(III) fluoride CoF 3, nitrate Co(NO 3) 3, and sulfate Co 2(SO 4) 3; however, cobalt(III) chloride...
21 KB (2,143 words) - 16:12, 22 March 2025
Manganese(III) fluoride (also known as Manganese trifluoride) is the inorganic compound with the formula MnF3. This red/purplish solid is useful for converting...
10 KB (778 words) - 16:34, 11 April 2025
fluoride is so high, +2.87 V, cobalt(III) fluoride is one of the few simple stable cobalt(III) compounds. Cobalt(III) fluoride, which is used in some fluorination...
122 KB (12,558 words) - 06:59, 16 May 2025
Thullium(III) fluoride is an inorganic compound with the chemical formula TmF3. It can be produced by reacting thulium(III) sulfide and hydrofluoric acid...
4 KB (280 words) - 17:58, 18 August 2022
contaminated products. The simplest method avoids fluorine gas but uses cobalt(III) fluoride: NO2 + CoF3 → NO2F + CoF2 The CoF2 can be regenerated to CoF3. Other...
4 KB (404 words) - 18:55, 20 December 2024
interests. Actinium(III) chloride – AcCl3 Actinium(III) fluoride – AcF3 Actinium(III) oxide – Ac2O3 Actinium(III) sulfide - Ac2S3 Actinium(III) nitrate - Ac(NO3)3...
121 KB (8,924 words) - 00:00, 18 May 2025
Cobalt compounds are chemical compounds formed by cobalt with other elements. Many halides of cobalt(II) are known: cobalt(II) fluoride (CoF2) which is...
16 KB (1,842 words) - 04:49, 4 April 2025
Lutetium(III) fluoride is an inorganic compound with a chemical formula LuF3. Lutetium(III) fluoride can be produced by reacting lutetium oxide with hydrogen...
4 KB (319 words) - 22:07, 23 December 2024
magnesium, and fluorinating agents such as xenon difluoride and cobalt(III) fluoride. At temperatures above 650–700 °C (1,200–1,290 °F) PTFE undergoes...
59 KB (6,073 words) - 20:02, 20 May 2025
is manufactured by the fluorination of tetralin or decalin with cobalt(III) fluoride in the Fowler process. For most applications, several steps of purification...
8 KB (717 words) - 15:31, 13 April 2025
Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal...
6 KB (466 words) - 16:16, 5 May 2025
hydrogen fluoride at 500 °C. Sodium hexafluorosilicate under pressure at 270 °C, titanium(IV) fluoride, chlorine trifluoride, cobalt(III) fluoride, iodine...
13 KB (1,108 words) - 08:06, 17 April 2025
Nickel(III) fluoride is the chemical compound with the formula NiF3. It is an ionic compound of nickel and fluorine. Nickel(III) fluoride can be prepared...
2 KB (68 words) - 09:37, 19 March 2025
of fluoride vapors emitted from cobalt(III) fluoride or cerium(IV) fluoride. It can be obtained by reacting terbium(III) chloride or terbium(III) fluoride...
44 KB (5,444 words) - 14:32, 27 April 2025
Fluoride toxicity is a condition in which there are elevated levels of the fluoride ion in the body. Although fluoride is safe for dental health at low...
26 KB (2,716 words) - 09:32, 7 April 2025
13455-25-9 CoF2 cobalt(II) fluoride 10026-17-2 CoF3 cobalt(III) fluoride 10026-18-3 Co(IO3)2 cobalt(II) iodate 13455-28-2 CoI2 cobalt(II) iodide 15238-00-3...
183 KB (107 words) - 23:35, 7 May 2025
Copper(II) fluoride or cupric fluoride is an inorganic compound with the chemical formula CuF2. The anhydrous form is a white, ionic, crystalline, hygroscopic...
11 KB (963 words) - 02:09, 6 March 2025
form melts at 678 °C. At higher temperatures, cobalt(II) bromide reacts with oxygen, forming cobalt(II,III) oxide and bromine vapor. The tetrahydrate is...
6 KB (351 words) - 05:19, 13 January 2025
americium(III) fluoride (AmF3), and curium(III) fluoride (CmF3). The mixture of UF6 and NpF6 is then selectively reduced by pelleted cobalt(II) fluoride, which...
13 KB (1,316 words) - 11:01, 18 March 2025
10241–04–0 CoCrO4 cobalt(II) chromate 24613–38–5 CoCr2O4 cobalt(II) chromite 13455–25–9 CoF2 cobalt(II) fluoride 10026–17–2 CoF3 cobalt(III) fluoride 10026–18–3...
139 KB (120 words) - 17:15, 19 May 2025
Fluoride batteries (also called fluoride shuttle batteries) are a rechargeable battery technology based on the shuttle of fluoride, the anion of fluorine...
22 KB (2,317 words) - 17:03, 22 May 2025
Iron(II) fluoride or ferrous fluoride is an inorganic compound with the molecular formula FeF2. It forms a tetrahydrate FeF2·4H2O that is often referred...
12 KB (991 words) - 02:47, 14 April 2025
deposited in fumaroles. Fluoride sulfates were first discovered by Jean Charles de Marignac in 1859. Some elements such as cobalt or uranium can form complexes...
50 KB (2,685 words) - 14:51, 22 May 2025