Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are hygroscopic blue solids...
15 KB (1,334 words) - 17:36, 17 November 2024
Copper(I) nitrate is a proposed inorganic compound with formula of CuNO3. It has not been characterized by X-ray crystallography. It is the focus of one...
3 KB (215 words) - 20:34, 12 October 2024
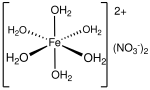
Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however...
8 KB (733 words) - 15:49, 11 March 2025
Nickel (II) nitrate is the inorganic compound Ni(NO3)2 or any hydrate thereof. In the hexahydrate, the nitrate anions are not bonded to nickel. Other hydrates...
6 KB (392 words) - 19:06, 5 March 2025
copper(II) oxide is conveniently prepared by pyrolysis of copper(II) nitrate or basic copper(II) carbonate: 2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2 (180°C) Cu2(OH)2CO3...
10 KB (832 words) - 03:37, 2 June 2025
(II) salts) are often blue to green in color, rather than the orange color copper is known for. Despite being considered a semi-noble metal, copper is...
8 KB (101 words) - 09:36, 4 August 2024
non-explosive products, these being nitrogen, nitrogen oxides and copper(II) nitrate. Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.)...
3 KB (127 words) - 15:00, 29 October 2023
following the element name. For example, Cu(NO3)2 is copper(II) nitrate, because the charge of two nitrate ions (NO− 3) is 2 × −1 = −2, and since the net charge...
13 KB (1,468 words) - 15:58, 27 September 2024
Cu(NH3)2(C3N3O3)2 can be prepared by heating copper(II) compounds, such as copper(II) nitrate or basic copper carbonate, with molten urea up to 190°C. 6...
6 KB (477 words) - 01:20, 28 May 2025
nitric acid produce copper(II) sulfate and copper(II) nitrate, respectively.[page needed] In terms of their coordination spheres, copper centres are 2-coordinated...
13 KB (1,104 words) - 04:31, 8 June 2025
acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the...
14 KB (1,494 words) - 04:48, 4 April 2025
active copper chromite catalyst which includes barium in its structure can be prepared from a solution containing barium nitrate, copper(II) nitrate, and...
10 KB (999 words) - 19:11, 29 April 2025
acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the...
126 KB (14,396 words) - 19:29, 9 June 2025
calculations of an oxalato-bridged copper(II) complex: μ-oxalato-bis[(2,2'-bipyridine) hydrate copper(II) nitrate]". Journal of the Iranian Chemical Society...
39 KB (2,151 words) - 13:30, 22 May 2025
– Cu(OH)2 Copper(II) nitrate – Cu(NO3)2 Copper(II) oxide – CuO Copper(II) perchlorate – Cu(ClO4)2 Copper(II) phosphate – Cu3(PO4)2 Copper(II) selenide...
121 KB (8,924 words) - 03:42, 25 May 2025
triphenylphosphine) Given nitrate's low basicity, the tendency of metal nitrate complexes toward hydrolysis is expected. Thus copper(II) nitrate readily dissociates...
11 KB (1,185 words) - 15:04, 11 April 2025
Nitric acid (redirect from Hydrogen nitrate)
industry) with either sulfuric acid or magnesium nitrate. Alternatively, thermal decomposition of copper(II) nitrate gives nitrogen dioxide and oxygen gases;...
47 KB (5,200 words) - 15:49, 15 May 2025
diazonium salt. In the presence of copper catalyst, such as copper(I) oxide, and an excess of copper(II) nitrate, this reaction takes place readily at...
16 KB (1,738 words) - 11:35, 22 April 2025
Salser (1973). "Mass spectra of covalent inorganic nitrates: copper(II) nitrate and titanium(IV) nitrate". Journal of Inorganic and Nuclear Chemistry. 35...
9 KB (765 words) - 23:24, 8 June 2025
Nitrate is a polyatomic ion with the chemical formula NO− 3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers...
38 KB (4,301 words) - 20:56, 9 June 2025
Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2. This colorless, crystalline salt is highly deliquescent. It is typically encountered...
5 KB (411 words) - 07:52, 14 March 2025
aqueous copper(II) nitrate (Cu(NO3)2) and solid silver. How much silver is produced if 16.00 grams of Cu is added to the solution of excess silver nitrate? The...
36 KB (5,402 words) - 09:07, 11 June 2025
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally...
6 KB (723 words) - 16:57, 3 January 2025
needed] Mercury(II) nitrate and potassium nitrate in water solution produce the salt tripotassium tetranitratomercurate(II) nitrate K3[Hg(NO2)4]NO3....
12 KB (831 words) - 21:50, 22 May 2025
Nitrate chlorides are mixed anion compounds that contain both nitrate (NO3−) and chloride (Cl−) ions. Various compounds are known, including amino acid...
65 KB (3,736 words) - 13:01, 22 May 2025
bidentate nitrate ligands. The Ag-O distances range from 2.384 to 2.702 Å. A typical reaction with silver nitrate is to suspend a rod of copper in a solution...
24 KB (2,345 words) - 22:54, 25 May 2025
acid, copper(II) nitrate, and nitric acid to change the color of the steel from silver-grey to blue-grey or black. Alternative procedures use copper sulfate...
9 KB (568 words) - 23:26, 9 January 2025
Copper(II) iodate Cu(IO3)2·2H2O 0.109 Copper(II) nitrate Cu(NO3)2 83.5 100 125 156 163 182 208 222 247 Copper oxalate CuC2O4·2H2O 2.1627×10−10 Copper(II)...
83 KB (225 words) - 19:01, 4 May 2025
Copper electroplating is the process of electroplating a layer of copper onto the surface of a metal object. Copper is used both as a standalone coating...
24 KB (2,640 words) - 03:46, 2 June 2025
"Hexaaquacobalt(II) nitrate". Cryst. Struct. Commun. 2 (4): 581–583. Gallezot, P.; Weigel, D.; Prettre, M. (1967). "Structure du Nitrate de Nickel Tétrahydraté"...
49 KB (3,030 words) - 23:51, 25 May 2025