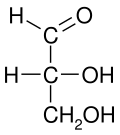
the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer...
11 KB (1,397 words) - 06:21, 1 April 2025
Structural formula (redirect from Sawhorse projection)
formulas, skeletal formulas, Newman projections, Cyclohexane conformations, Haworth projections, and Fischer projections. Several systematic chemical naming...
21 KB (2,563 words) - 00:35, 25 May 2025
below the plane of the ring in Haworth projections correspond to those on the right-hand side of a Fischer projection. This rule does not apply to the groups...
3 KB (310 words) - 10:45, 26 February 2025
or "L-", depending on whether the hydroxyl in position 5, in the Fischer projection of the molecule, is to the right or to the left of the axis, respectively...
16 KB (1,905 words) - 00:39, 20 March 2025
This projection is named after American chemist Melvin Spencer Newman, who introduced it in 1952 as a partial replacement for Fischer projections, which...
5 KB (548 words) - 06:18, 7 February 2024
regular chiral atoms. See: Fischer projection Construction of the Fischer projection D-glucose in the Fischer projection. Red: Group with highest priority...
22 KB (2,422 words) - 11:02, 26 February 2025
Nobel Prize in Chemistry. He discovered the Fischer esterification. He also developed the Fischer projection, a symbolic way of drawing asymmetric carbon...
18 KB (1,777 words) - 20:48, 24 May 2025
Monosaccharide (section Haworth projection)
bounded by 2c, where c is the total number of chiral carbons. The Fischer projection is a systematic way of drawing the skeletal formula of an acyclic...
16 KB (2,119 words) - 11:34, 22 April 2025
with all of its hydroxyl functional groups on the same side in its Fischer projection. d-Ribose has these hydroxyl groups on the right hand side and is...
32 KB (3,197 words) - 03:03, 23 April 2025
carbon in the chain. Aldoses with alcohol groups on the right of the Fischer projection are D-aldoses, and those with alcohols on the left are L-aldoses....
6 KB (631 words) - 16:22, 19 February 2025
in α-D-glucopyranose the reference atom is C-5. If in the cyclic Fischer projection the exocyclic oxygen atom at the anomeric centre is cis (on the same...
9 KB (1,067 words) - 03:53, 9 May 2025
convention. A Fischer projection can be used to differentiate between L- and D- molecules Chirality (chemistry). For instance, by definition, in a Fischer projection...
15 KB (1,640 words) - 04:24, 18 April 2025
drawing. Other types of representation, such as Newman projection, Haworth projection or Fischer projection, also look somewhat similar to skeletal formulae...
28 KB (3,571 words) - 06:25, 1 April 2025
asymmetric carbon furthest from the carbonyl group: in a standard Fischer projection if the hydroxyl group is on the right the molecule is a D sugar, otherwise...
57 KB (5,901 words) - 19:32, 31 May 2025
Wedge-and-dash notation in skeletal formulas Haworth projection Newman projection Fischer projection Dietrich Braun; Harald Cherdron; Matthias Rehahn; H. Ritter;...
2 KB (145 words) - 17:27, 29 October 2023
inventor of the Fischer projection Horst Fischer (1912–1966), German SS concentration camp doctor executed for war crimes Howard Fischer, curator of the...
11 KB (1,322 words) - 23:06, 31 May 2025
acid can exist as any of three stereoisomers depicted below in a Fischer projection. Of the four colored pictures at the top of the diagram, the first...
5 KB (607 words) - 18:36, 24 June 2024
three-dimensional object Fischer projection, a two-dimensional representation of a three-dimensional organic molecule Haworth projection, a way of writing a...
4 KB (468 words) - 13:14, 23 January 2025
designate the relative configuration of the centers". As is depicted in a Fischer projection of d-threose, the adjacent substituents will have a syn orientation...
4 KB (288 words) - 22:22, 12 May 2025
absolute configuration of some carbohydrates and amino acids according to Fischer projection (D/L system) and Cahn–Ingold–Prelog priority rules (R/S system)...
16 KB (1,799 words) - 20:16, 23 April 2025
as the Calvin cycle. The intermediates of glycolysis depicted in Fischer projections show the chemical changing step by step. Such image can be compared...
82 KB (8,731 words) - 04:19, 25 May 2025
yield the same aldaric acid (this can be understood by looking at the Fischer projection of a sugar upside down—with normal aldoses, this is a different compound...
4 KB (502 words) - 03:55, 26 February 2024
in deoxyribose all the hydroxyl groups are on the same side in the Fischer projection. The term "2-deoxyribose" may refer to either of two enantiomers:...
7 KB (710 words) - 06:04, 9 February 2025
unambiguous descriptions of their relative positions in the molecule. A Fischer projection is a simplified way to depict the stereochemistry around a stereocenter...
12 KB (1,263 words) - 22:18, 24 May 2025
l-Glucose Haworth projection of α-l-glucopyranose Fischer projection of l-glucose Names IUPAC name l-Glucose Identifiers CAS Number 921-60-8 Y 3D model...
5 KB (408 words) - 00:27, 25 May 2025
threo and erythro. In the case of saccharides, when drawn in the Fischer projection the erythro isomer has two identical substituents on the same side...
10 KB (1,254 words) - 12:25, 8 May 2025
relative stereochemistry of the anomeric (or most oxidized) carbon. In a Fischer Projection, if the glycosidic linkage is on the same side or face as carbon 6...
10 KB (1,107 words) - 05:27, 3 June 2024
same molecular graph may exist as two or more stereoisomers. The Fischer projection is a systematic way of drawing the skeletal formula of an open-chain...
13 KB (1,150 words) - 17:03, 18 January 2025
of glycerol (2-sn) is on the left on a Fischer projection. The numbering follows the one of Fischer's projections, being 1-sn the carbon at the top and...
18 KB (2,269 words) - 00:57, 24 May 2025