molar mass constant, usually denoted by Mu, is a physical constant defined as one twelfth of the molar mass of carbon-12: Mu = M(12C)/12. The molar mass...
7 KB (1,090 words) - 16:46, 30 December 2024
In chemistry, the molar mass (M) (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical compound is...
21 KB (3,223 words) - 12:10, 20 April 2025
Dalton (unit) (redirect from Atomic mass constant)
as well. As a consequence, the molar mass constant remains close to but no longer exactly 1 g/mol, meaning that the mass in grams of one mole of any substance...
36 KB (3,870 words) - 22:42, 11 May 2025
molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R or R. It is the molar equivalent...
17 KB (1,839 words) - 00:58, 15 April 2025
Avogadro constant NA is also the factor that converts the average mass ( m {\displaystyle m} ) of one particle, in grams, to the molar mass ( M {\displaystyle...
20 KB (2,297 words) - 17:01, 27 April 2025
times its molar mass. The SI unit of molar heat capacity is joule per kelvin per mole, J⋅K−1⋅mol−1. Like the specific heat, the measured molar heat capacity...
50 KB (5,707 words) - 19:29, 21 March 2025
mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided...
12 KB (1,482 words) - 06:54, 20 April 2025
{\displaystyle M_{\rm {u}}} is the molar mass constant, N A {\displaystyle N_{\rm {A}}} is the Avogadro constant, and M ( 12 C ) {\displaystyle M(^{12}\mathrm...
22 KB (2,778 words) - 19:04, 20 May 2025
^{2}}},} where Mu is the molar mass constant (defined in SI); Ar(e) is a directly measured quantity, the relative atomic mass of the electron. mu is defined...
10 KB (1,493 words) - 09:08, 13 January 2025
Value: molar mass constant". The NIST Reference on Constants, Units, and Uncertainty. NIST. May 2024. Retrieved 2024-05-18. "2022 CODATA Value: molar volume...
32 KB (1,713 words) - 16:38, 26 September 2024
Molar concentration (also called molarity, amount concentration or substance concentration) is the number of moles of solute per liter of solution. Specifically...
11 KB (1,418 words) - 03:00, 15 May 2025
Amount of substance (redirect from Molar quantity)
historically defined such that the molar mass constant was exactly 1 g/mol. Thus, given the molecular mass or formula mass in daltons, the same number in...
29 KB (3,530 words) - 17:45, 23 May 2025
to the molar mass (M) divided by the mass density (ρ): V m = V n = M ρ {\displaystyle V_{\text{m}}={\frac {V}{n}}={\frac {M}{\rho }}} The molar volume...
6 KB (855 words) - 17:00, 9 August 2024
In chemistry, the molar absorption coefficient or molar attenuation coefficient (ε) is a measurement of how strongly a chemical species absorbs, and thereby...
6 KB (760 words) - 15:51, 19 January 2025
Mole (unit) (redirect from Gram molecular mass)
may be specified. The mass of a substance is equal to its relative atomic (or molecular) mass multiplied by the molar mass constant, which is almost exactly...
27 KB (3,413 words) - 14:21, 3 May 2025
is the ideal gas constant. M is the molar mass of the solvent. Tb is boiling point of the pure solvent in kelvin. ΔHvap is the molar enthalpy of vaporization...
3 KB (261 words) - 07:45, 19 March 2024
molality of the solution. Through cryoscopy, a known constant can be used to calculate an unknown molar mass. The term "cryoscopy" means "freezing measurement"...
2 KB (300 words) - 12:09, 19 March 2024
Solubility equilibrium (redirect from Molar solubility)
is known as the solubility. Units of solubility may be molar (mol dm−3) or expressed as mass per unit volume, such as μg mL−1. Solubility is temperature...
29 KB (4,103 words) - 20:14, 13 February 2025
Gravitational constant: G = 6.67430(15)×10−11 m3⋅kg−1⋅s−2 Molar mass constant: Mu = 1.00000000105(31)×10−3 kg⋅mol−1 Planck constant: h = 6.62607015×10−34 J⋅Hz−1...
58 KB (3,970 words) - 17:17, 23 May 2025
2019 revision of the SI (redirect from SI defining constant)
remains valid, which means the second is no longer exactly true. The molar mass constant, while still with great accuracy remaining 1 g/mol, is no longer...
76 KB (9,060 words) - 06:03, 14 May 2025
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion...
14 KB (1,697 words) - 07:04, 19 April 2025
Specific heat capacity (redirect from Molar specific heat)
per mass equations: C p , m = ( ∂ C ∂ n ) p = molar heat capacity at constant pressure C V , m = ( ∂ C ∂ n ) V = molar heat capacity at constant volume...
55 KB (8,537 words) - 22:17, 8 April 2025
ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constant. The atomic mass constant (symbol: mu) is defined...
18 KB (2,331 words) - 05:20, 15 January 2025
their efforts to refine experiments that link the unit of mass to fundamental or atomic constants with a view to a future redefinition of the kilogram"....
13 KB (803 words) - 17:25, 16 September 2024
{\displaystyle C_{P,\mathrm {m} }^{\circ }} is the standard molar heat capacity of a substance at constant pressure. Δ r H m ∘ {\displaystyle \mathrm {\Delta }...
20 KB (2,466 words) - 08:54, 19 February 2025
is molar absorptivity. They are quantitatively related by (mass attenuation coefficient) × (molar mass) = (molar absorptivity). Tables of photon mass attenuation...
8 KB (909 words) - 22:23, 15 April 2025
Mole fraction (redirect from Molar fraction)
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount...
10 KB (1,837 words) - 15:51, 19 January 2025
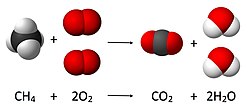
Stoichiometry (redirect from Mass ratio (mixtures))
R = universal ideal gas law constant T = absolute gas temperature ρ = gas density at T and P m = mass of gas M = molar mass of gas In the combustion reaction...
36 KB (5,365 words) - 13:14, 14 May 2025
Z its atomic number, A its relative atomic mass, NA the Avogadro number and Mu the Molar mass constant. In the figure to the right, the small circles...
8 KB (947 words) - 05:53, 26 April 2025
engineering materials, and (when applicable) the molar heat capacity. Generally, the most notable constant parameter is the volumetric heat capacity (at...
16 KB (830 words) - 16:23, 19 May 2025